Clostridium Difficile Diagnostics and Treatment Market – Insights
Clostridium difficile is a disease caused by bacteria. Symptoms of the disease includes diarrhea, nausea, colon inflammation, and others. Clostridium difficile infection affects older adults, and according to Centers for Disease Control and Prevention (CDC), 2015, clostridium difficile infection is the most common healthcare-associated infection. Furthermore, frequent use of antibiotic medication is also responsible for clostridium difficile infection, as antibiotics kill normal flora (good bacteria) and allow for multiplication of C. difficile bacteria. Common symptoms of clostridium difficile infection include watery diarrhea, dehydration, fever, nausea, loss of appetite, and weight loss. Life threatening symptoms of the disease includes abdominal distension, severe abdominal pain, and profuse diarrhea. Clostridium difficile infection management includes diagnosis of the infection using various tests and medication therapies using antibiotics such as Vancomycin and Fidaxomicin.
Rising prevalence of clostridium difficile infection is expected to boost growth of the market over the forecast period
Incidence of clostridium difficile infection has been witnessing an increase in the recent past, due to clostridium difficile infection recurrence and antibiotic resistance. According to Centers for Disease Control and Prevention (CDC), 2015, around half a million people in the U.S. suffer from clostridium difficile infection in a year. Moreover, according to National Center for Biotechnology Information (NCBI), an estimated 37,900 patients were suffering from clostridium difficile infection in Canada in 2012. A review and meta-analysis of studies published in National Center for Biotechnology Information (NCBI), 2016, conducted in China between 2010 and 2016 showed significant incidence of clostridium difficile infection in China. Furthermore, according to Canadian Antimicrobial Resistance Surveillance System 2017 Report, clostridium difficile infection may be a consequence of commonly prescribed antibiotics for unrelated infections. According to Organization for Economic Co-operation and Development (OECD), 2015, antibiotic resistant bacteria are highly prevalent in G7 countries. This high presence of antibiotic resistant bacteria may lead to increasing incidence of the infection, which in turn is expected to aid in growth of the global clostridium difficile diagnostics and treatment market.
The global clostridium difficile diagnostics and treatment market was valued at US$ 1,272.2 million in 2016 and is expected to witness a robust CAGR of 8.2% over the forecast period (2017–2025).
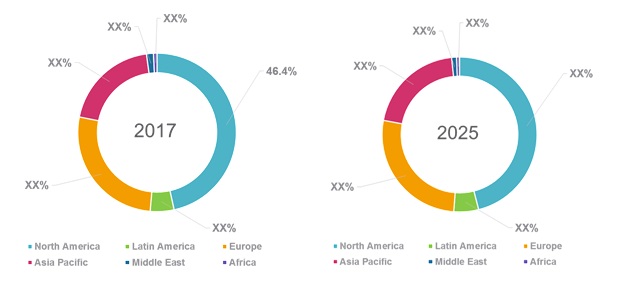
Figure 1. Global Clostridium Difficile Diagnostics and Treatment Market Share (%) 2017 and 2025, by Region
To learn more about this report, Download Free Sample
Robust pipeline of various therapies is expected to boost growth of the clostridium difficile treatment market over the forecast period
Standard antibiotic drugs such as metronidazole, Vancomycin, and Fidaxomicin have been used for the treatment of clostridium difficile infection (CDI) since the recent past. Currently, there is no alternative therapies apart from antibiotics available for the treatment of clostridium difficile infection and only option available is surgery (in severe cases), fecal microbiota transplant, and probiotics in case of recurrent clostridium difficile infection. However, Manufacturers such as Rebiotix, Inc., Synthetic Biologics, Inc., Da Volterra, Crestovo LLC, and Immuron Ltd. have various microbiome therapeutics (new class of drugs) in pipeline for clostridium difficile infection indication. Rebiotix, Inc. has RBX7455 capsule in Phase 1 clinical trials, Synthetic Biologics Inc. has SYN-004 (ribaxamase) in Phase 2 clinical trials, and Da Volterra has DAV132 in clinical trials for antibiotic induced clostridium difficile infection. If these drugs show success in clinical trials, it could provide an alternative therapy for conventional antibiotics for treatment of the disease. Furthermore, probiotics are also gaining traction as they allow growth of natural flora through restoring natural balance of bacteria in the gut. Manufacturers such as Pfizer, Inc. and Valneva are working on developing novel vaccines for the treatment of CDI. For instance, Pfizer, Inc. has recombinant TcdA and TcdB PF-06425090 vaccine in phase 2 trial and Valneva has VLA84 in phase 1 trial.
However, low awareness about clostridium difficile infection symptoms and associated effects is expected to restrain the growth of the clostridium difficile diagnostics and treatment market over the forecast period.
Some major players operating in the clostridium difficile diagnostics and treatment market include Roche AG, Thermo Fisher Scientific Inc., Merck & Co., Pfizer, Inc., Actelion Pharmaceuticals, Alere, Inc., Trinity Biotech, Summit Therapeutics, Baxter International Inc., Sanofi S.A., Novartis AG, and AstraZeneca Plc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients