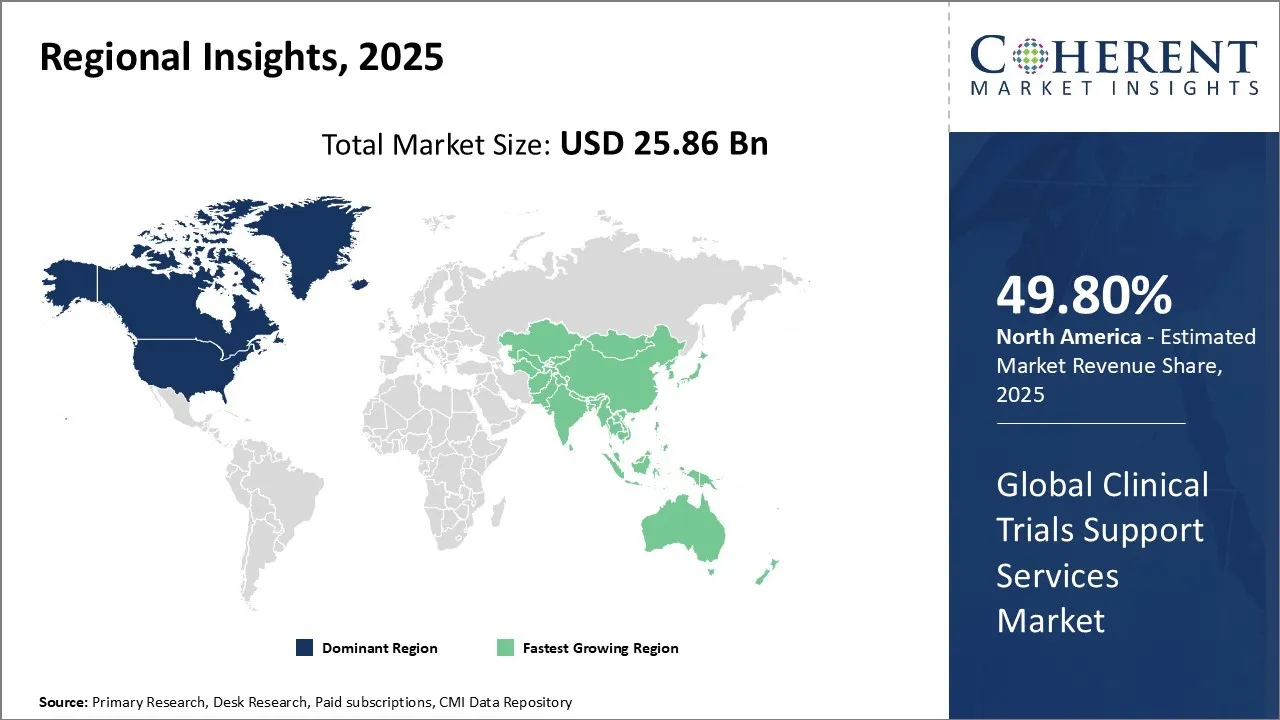
Clinical Trials Support Services Market is estimated to be valued at USD 25.86 Bn in 2025 and is expected to reach USD 42.9 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of7.5% from 2025 to 2032.
The global clinical trials support services market is experiencing strong growth owing to the increasing demand for clinical trials support services and rise in burden of chronic and infectious disease. Furthermore, increasing number of clinical trials and increase in drug discovery and drug development is expected to boost the clinical trials support services market growth.
|
Current Events |
Description and its impact |
|
Geopolitical and Regulatory Shifts |
|
|
Technological Innovations and Digital Transformation |
|
|
Economic Fluctuations and Funding Dynamics |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Phase III acquired the prominent share of 54.2% in 2025. Growing rates of chronic diseases, rising drug development activity, and increasing trial complexity drive the Phase 3 Clinical Trials Support Services market. Pharmaceutical and biotech companies actively outsource services to improve efficiency, meet regulatory requirements, and cut operational costs. They also adopt technologies like electronic data capture and remote monitoring to streamline trial processes. As the demand for large, diverse patient populations grows, these companies expand trials globally, further increasing their reliance on comprehensive support services during this pivotal phase. For instance, in December 2024, EyePoint Pharmaceuticals, Inc., focused on developing innovative treatments for serious retinal diseases, has announced that it has dosed the first patient in the LUCIA trial—its second global Phase 3 clinical trial of DURAVYU (formerly EYP-1901) for treating wet AMD.
Increasing trial complexity, rising pressure to recruit patients efficiently, and the need to meet regulatory standards drive growth in the Clinical Trial Site Management segment. Pharmaceutical and biotech companies actively outsource site management to improve operational efficiency, lower costs, and maintain quality control. They adopt digital tools like clinical trial management systems and remote monitoring to optimize site performance. As they expand trials into emerging markets, they rely more on skilled site management teams to coordinate and support studies across multiple regions. For instance, in March 2025, Neutra Corp entered the Life Sciences clinical research sector through its pilot program and announced the launch of its advanced Site Management Organization (SMO), Neutra Life Sciences.
Pharmaceutical and biopharmaceutical companies actively fuel the Clinical Trials Support Services market by boosting research and development efforts and speeding up drug discovery. They increasingly outsource support services to cut costs, enhance efficiency, and leverage specialized expertise. As they shift toward personalized medicine and manage more complex trial designs, they seek tailored solutions. These companies also embrace digital innovations and expand their trials globally, driving the need for comprehensive support services to ensure smooth and successful trial execution across diverse regions.
In February 2025, Selkirk Pharma, Inc., a privately held U.S. pharmaceutical manufacturer focused on fill and finish services for injectable drugs, launched ClinFAST™—a groundbreaking service aimed at helping clinical-stage biotech and pharma companies quickly complete the fill/finish process for their clinical trial supplies.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 49.80% in 2025. Advanced healthcare infrastructure, strong R&D activity, and the presence of major pharmaceutical and biotech companies are driving growth in the North America Clinical Trials Support Services market. Industry players in the region actively adopt innovative technologies like electronic data capture, remote monitoring, and decentralized trial models to enhance trial efficiency. Regulatory agencies provide clear guidance and streamlined approvals, encouraging market expansion. Companies also prioritize patient-centric strategies and increasingly outsource trial operations, which continues to shape evolving market trends across the United States and Canada. For instance, in December 2024, USF Health unveiled a state-funded hyperbaric oxygen therapy trial, enrolling its first patient. National experts recognize this ambitious project as one of the most rigorous studies of its kind, with long-term results that could transform the understanding of hyperbaric oxygen therapy as a treatment for neurological diseases.
Clinical trial activity is rapidly increasing in Asia Pacific as countries like China, India, and South Korea attract attention with their large, diverse patient populations and growing disease burden. Global pharmaceutical companies are choosing these locations for their cost-efficiency and expanding healthcare systems. Governments and private investors across the region are actively building healthcare infrastructure and upgrading clinical research facilities. These advancements enable the region to offer modern trial sites and skilled professionals, making it an increasingly attractive hub for international clinical research. For instance, in July 2025, Intrinseque Health innaugrated its new entity, Intrinseque Health (Malaysia) Sdn. Bhd. This entity will strengthen operations across Southeast Asia, supporting drug development, clinical trials, and clinical supply chain activities throughout the region.
Sponsors and research organizations in the U.S. are actively implementing decentralized clinical trial models to enhance patient participation and minimize the need for site visits. By using remote monitoring, telehealth, and digital platforms, they improve recruitment and retention across diverse regions. U.S. pharmaceutical and biotech companies increasingly outsource trial operations to Contract Research Organizations (CROs) and Site Management Organizations (SMOs). This strategy helps them reduce costs, accelerate timelines, and access expert support for managing complex, multi-site, and multi-phase clinical trials.
For instance, in May 2025, U.S. based company, OptymEdge, a part of the Emmes Group, expanded its ophthalmology services by launching the clinical research industry’s first technology platform specifically designed for ophthalmology trials.
Global pharmaceutical and biotech companies are increasingly choosing India as a key destination for clinical trials, drawn by its large patient pool, diverse disease patterns, and growing involvement in international research. Sponsors are conducting more studies in India to achieve faster patient enrollment and reduce costs. They are actively outsourcing trial support services to Indian Contract Research Organizations (CROs) and Site Management Organizations (SMOs), which provide experienced personnel, flexible operations, and affordable solutions, positioning India as a strategic hub for clinical trial outsourcing. For instance, in April 2024, the Indian Council of Medical Research (ICMR) announced tp expand its Indian Clinical Trial and Education Network (INTENT) to conduct large, regulation-compliant clinical trials aimed at delivering evidence-based, scientifically robust, and culturally appropriate solutions to health challenges of national importance.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 25.86 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.5% | 2032 Value Projection: | USD 42.9 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Wuxi AppTec, Inc., IQVIA Holdings, Inc., Syneos Health, Inc., Eurofins Scientific, Laboratory Corporation of America Holdings (Labcorp), Icon PLC, ALCURA, Parexel International Corporation, PPD, Inc. (Pharmaceutical Product Development), and Charles River Laboratories International, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The market is witnessing a strong shift toward decentralized clinical trials, driven by the need for improved patient accessibility and operational flexibility. DCTs use technologies like telemedicine, remote monitoring, and wearable devices to conduct trials outside traditional sites. This approach reduces patient burden, increases engagement, and expands geographic reach. Sponsors are investing in service providers that can support hybrid and remote models, making decentralized trial capabilities a major competitive differentiator in the clinical trials support services landscape.
Pharmaceutical and biotech companies are increasingly outsourcing clinical trial activities to Contract Research Organizations (CROs) and Site Management Organizations (SMOs). This trend is driven by the need to reduce costs, improve trial efficiency, and access specialized expertise. CROs offer end-to-end support, while SMOs focus on site-level coordination and recruitment. Outsourcing allows sponsors to focus on core R&D while delegating operations, compliance, and monitoring tasks to experienced partners, fueling the demand for outsourced support services across all trial phases.
AI and automation present significant opportunities to streamline clinical trial processes. Service providers can leverage AI for patient matching, protocol design, risk-based monitoring, and predictive analytics. Automation reduces manual errors and accelerates data processing. Companies that invest in AI-driven platforms can offer faster, more accurate services and gain a competitive edge. As trials become more complex, the demand for intelligent, data-driven decision-making tools creates a vast opportunity for innovation in support services.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients