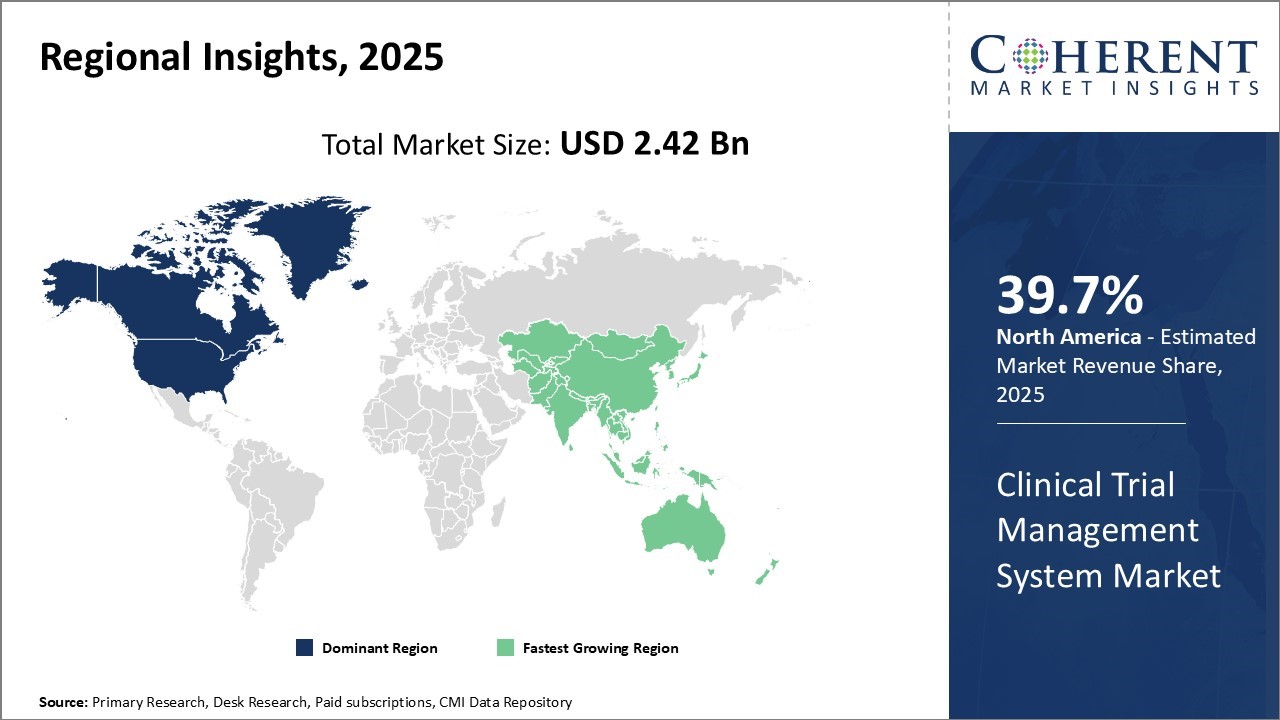
The clinical trial management system market is estimated to be valued at USD 2.42 Bn in 2025 and is expected to reach USD 6.47 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 15.1% from 2025 to 2032.
To learn more about this report, Download Free Sample
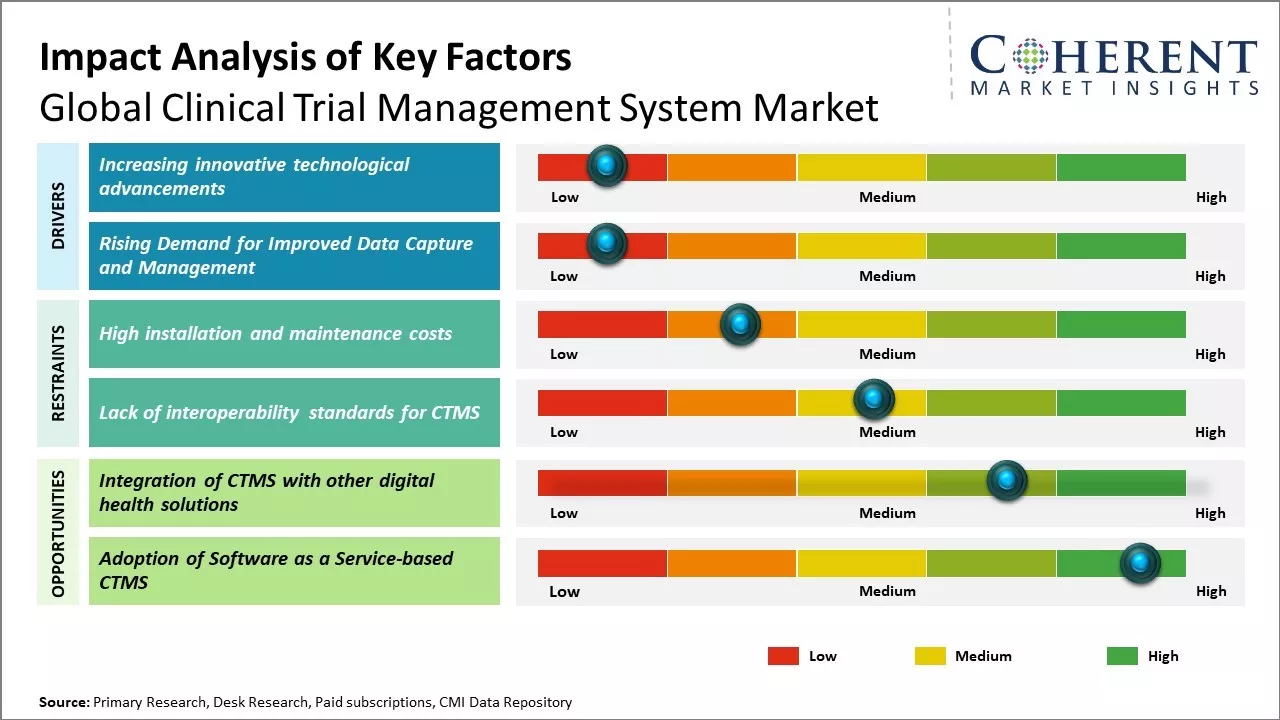
The clinical trial management system market is expected to witness significant growth over the forecast period. There is an increase in the number of clinical trials conducted across the globe on a yearly basis. In addition, rising R&D expenditure of pharmaceutical and biotechnology companies for developing new drug molecules is also supporting the growth of this market. Furthermore, increasing outsourcing of clinical trials to CROs coupled with rising adoption of clinical trial management system to maintain compliance and improve the efficiency of trials will further aid in market expansion. Various advantages offered by CTMS software over manual processing such as expense tracking, timely report generation, monitoring performance indicators, and reducing redundancy are further driving the demand for these systems.
|
Current Events |
Description and its impact |
|
EU Clinical Trials Regulation Full Implementation and Regulatory Harmonization |
|
|
Decentralized Clinical Trials Regulatory Endorsement and Market Transformation |
|
|
US Government Clinical Trial Infrastructure Modernization Initiative |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
AI plays a crucial role in Clinical Trial Data Management Systems by streamlining data handling and improving accuracy. It automates the extraction and integration of both structured and unstructured data from sources like electronic health records, reducing manual errors. AI-powered tools clean and validate data, ensuring higher quality for analysis. Additionally, AI enhances patient recruitment by matching participants to trial criteria more efficiently. Natural Language Processing (NLP) helps extract valuable insights from unstructured clinical notes.
In April 2025, Axtria Inc., an AI-driven life sciences analytics solutions, launched LUCCID (LLM-based Unstructured Clinical Concept Identification). This GenAI-powered solution transforms the extraction and processing of unstructured data from electronic health records (EHRs). Designed to complement FHIR-based EHR-to-EDC systems that handle structured data, LUCCID addresses the major challenge of manually extracting unstructured clinical trial data, which makes up 80% of all trial information.
On-premise is expected to contribute the highest share with 59.7% in 2025. On-premise CTMS offer consolidated systems that are extensively customized as per end user's extensive operational needs and databases. This allows integration of CTMS with other internal legacy systems and customized features specific to therapeutic areas and trial complexities. Large pharma and CROs have highly complex operational workflows involving management of global trials, investigator engagement, lab sample management, supply chain coordination and safety data analysis. On-premise CTMS configured exclusively for them provide a centralized platform to oversee all aspects of complex trials. Customized interfaces, role-based access, integration with LIMS, EDC and other internal apps ensure seamless data exchange. This centralized oversight and consolidated access to all trial data enhances efficiency. Additionally, on-premise systems give higher data security and control over sensitive patient data to organizations.
Software segment is expected to contribute the largest market share with 70.6% in 2025. CTMS software packages currently offer a comprehensive suite of features ranging from site selection and contracts, to patient enrollment tracking, lab data integration, supply chain management, and safety reporting. Pharma and CROs now prefer customizable CTMS platforms providing a centralized ‘paperless office’ solution eliminating disjointed excel sheets and legacy systems. Integrated software suite assists in overseeing global trials on a single platform with functionality for site randomization & payments, lab sample coding, document approvals, supply ordering, and integrated EDC capturing eCRF data. This has streamlined activities of clinical operations, monitors, and data managers on a single collaborative space. Rise of specialized CTMS providers focusing on niche therapeutic areas and biomarker-based trials has further expanded the available suite of integrated solutions.
For instance, in November 2024, Slope and LabConnect have introduced a bidirectional application programming interface (API) integration to serve the clinical trial industry.
The biopharmaceutical companies’ segment is expected to contribute the highest share in the overall clinical trial management system market with 40.5% in 2025. Biopharma has continuously expanded pipelines involving several biological moieties, rare/orphan indications, and biomarker-driven compounds. Successful development and commercialization of such complicated molecules requires robust trial planning frameworks, global investigator networks, and meticulous supply chain coordination. CTMS tailored for biopharma allow customized design of protocols involving novel biomarkers, specialized CRO engagement, and lab sample mapping across hundreds of sites worldwide. Their scalable global trial networks are efficiently managed through functionalities for translations, payments, cold chain compliance, and real-time safety reporting.
For instance, in April 2025, Veeva Systems has announced Veeva SiteVault CTMS, a clinical trial management system for research sites that integrates with SiteVault eISF and SiteVault eConsent, enabling sites to manage clinical trials comprehensively within a single platform. Such innovations are proliferating the clinical trial management system market revenue.
To learn more about this report, Download Free Sample
North America acquires the largest share of 39.7%. Biopharmaceutical companies and CROs in North America are driving the growth of the Clinical Trial Management System (CTMS) market by adopting cloud-based platforms, integrating AI to boost trial efficiency, and embracing decentralized trial models. They are actively investing in advanced CTMS solutions to meet regulatory requirements and enable real-time data monitoring.
Government initiatives supporting clinical research and increasing collaboration between sponsors and research sites are also fueling innovation, strengthening North America’s leadership in global clinical development. For instance, Lindus Health, known as the "anti-CRO" for conducting faster, more reliable clinical trials, has launched its comprehensive contract research organization (CRO) and technology solution specifically designed to support the execution of ophthalmology clinical trials. Also, Canada accounts for 4% of clinical trials worldwide and ranks fourth in the number of clinical trial sites. It leads the G7 in clinical trial productivity, measured by the number of trials relative to population size. In 2023, Canada ranked third globally for the total number of new clinical trials and fourth for the total number of ongoing active trials, further accelerating the clinical trial management system market share.
In the Asia-Pacific region, clinical trial teams are adopting CTMS platforms enhanced with AI and machine learning to optimize protocols, forecast recruitment success, and identify data irregularities. They also leverage mobile apps and remote monitoring tools to conduct decentralized and hybrid trials. Sponsors take advantage of the region’s large, diverse populations and lower costs to accelerate phase III recruitment. As a result, global pharmaceutical companies and CROs are increasingly outsourcing trials to countries like India, China, and Southeast Asia, driving CTMS adoption. For instance, Tata Consultancy Services (TCS), a leading global IT services, consulting, and business solutions provider, has launched Connected Clinical Trials (CCT), an innovative SaaS platform. This platform helps pharmaceutical companies transform patient engagement in clinical trials and enhance the efficiency and accountability of the clinical supply process.
In the U.S., CTMS providers actively integrate advanced technology to predict patient enrollment, refine trial protocols, and identify anomalies in real time. They automate routine processes and improve data accuracy, allowing sponsors and CROs to speed up trials and simplify operations. Trial teams use CTMS-enabled mobile apps, remote monitoring, and telemedicine tools to conduct decentralized and hybrid studies. These technologies expand access for rural populations, enhance data collection, and reduce the burden on trial participants.
In January 2024, BSI Life Sciences presented a demonstration of its newest client, Ocular Therapeutix, featuring its cloud-based Clinical Trial Management System.
India’s Clinical Trial Management System (CTMS) market is advancing quickly as clinical research activity expands, digital platform adoption rises, and global pharmaceutical companies increase their involvement. Indian CROs and research institutions are actively adopting CTMS solutions to strengthen trial oversight, meet regulatory standards, and streamline multi-site trial management. For instance, in March 2025, NeOnc Technologies Holdings, Inc., a clinical-stage medical biotechnology company, today announced a strategic partnership with CBCC Global Research (CBCC), a premier full-service clinical research organization (CRO). This collaboration will expand NeOnc’s clinical trial capabilities in India, facilitating the advancement of its development-stage neuro-oncology treatment. This is further proliferating the clinical trial management system market demand.
To learn more about this report, Download Free Sample
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.42 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.1% | 2032 Value Projection: | USD 6.47 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
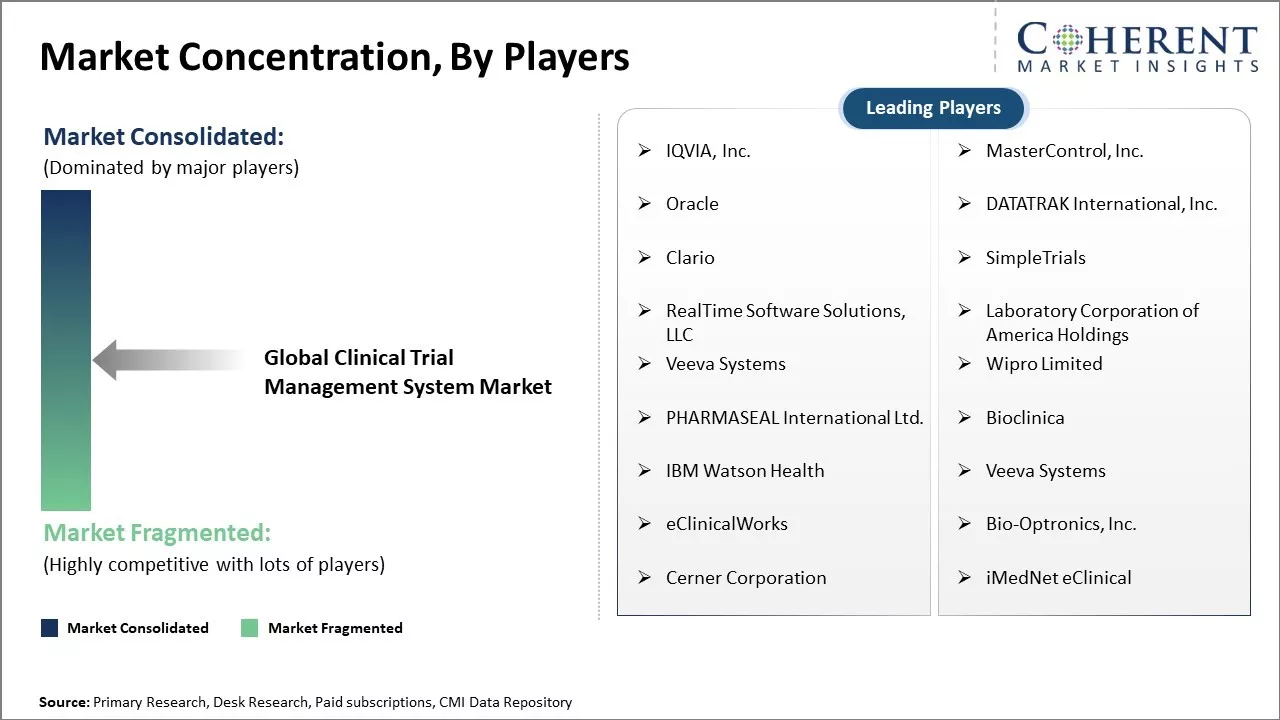
IQVIA, Inc., MasterControl, Inc., Oracle, DATATRAK International, Inc., Clario, SimpleTrials, RealTime Software Solutions, LLC, Laboratory Corporation of America Holdings, Veeva Systems, Wipro Limited, PHARMASEAL International Ltd., Bioclinica, IBM Watson Health, Veeva Systems, eClinicalWorks, Bio-Optronics, Inc., Cerner Corporation, and iMedNet eClinical |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Organizations increasingly shift toward cloud-based and SaaS CTMS platforms to enable centralized access, reduce infrastructure costs, and support remote work environments. These solutions provide real-time collaboration among sponsors, CROs, and research sites while improving data accessibility across geographically dispersed teams. Cloud-based CTMS also facilitates faster implementation, system scalability, and simplified upgrades, making it an attractive option for companies conducting global or multi-site trials.
The growing shift toward decentralized and hybrid clinical trial models is significantly influencing CTMS design and functionality. Sponsors and CROs now require platforms that integrate seamlessly with telemedicine, wearable devices, and remote data collection tools. This trend reflects a broader push for greater patient accessibility and participation, especially in remote or underserved locations, while also improving data timeliness and trial efficiency through digital monitoring and virtual site interactions.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients