Chronic Liver Diseases Therapeutics Market is estimated to be valued at USD 18.7 Bn in 2025 and is expected to reach USD 38.58 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 10.9% from 2025 to 2032.
Analysts’ Views on Global Chronic Liver Diseases Therapeutics Market:
Increasing prevalence of and awareness about chordoma disease, rising geratic population, and new drugs releases and strategic efforts by prominent market competitors are favorably impacting the market's growth. The high prevalence of cancer, favorable health reimbursement, and increased awareness regarding cancer among people and clinicians is a major factor in the chronic liver diseases therapeutics market, thereby contributing to the market growth.
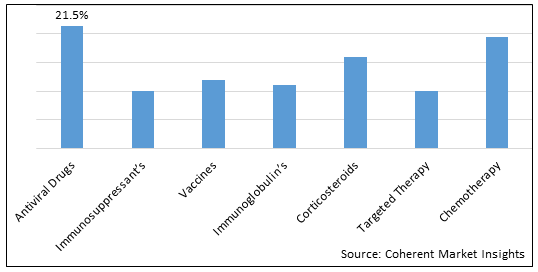
Figure 1. Global Chronic Liver Diseases Therapeutics Market Share (%), By Treatment Type, 2025
To learn more about this report, Download Free Sample
Global Chronic Liver Diseases Therapeutics Market– Driver
Increasing cancer cases such as liver cancer
Increasing cancer cases such as liver cancer is expected to propel growth of the global chronic liver diseases therapeutics market over the forecast period. For instance, according to an article published in the World Health Organization, in February 2022, the age-standardized point prevalence and annual incidence rates of cancer were 246.6 and 14.9 in 2021, which increased by 7.4% and 8.2% from 1990, respectively. Cancer is a leading cause of death worldwide, accounting for nearly 10 billion deaths in 2020. As per the national orgniasation of rare disorders reported, the incidence of liver is estimated approximately 1 per 1,000,000 people. About 300 cases of chordoma are diagnosed in the U.S. each year.
Increasing research & development activities for the treatment of chronic liver diseases
Increasing research & development activities for the treatment of chronic liver diseases is expected to drive growth of the global chronic liver diseases therapeutics market over the forecast period. For instance, in January 2022, Madrigal Pharmaceuticals, Inc. announced positive top line clinical data from the placebo-controlled, double-blind portion of its phase 3 MAESTRO-NAFLD (non-alcoholic fatty liver disease) safety study of resmetirom.
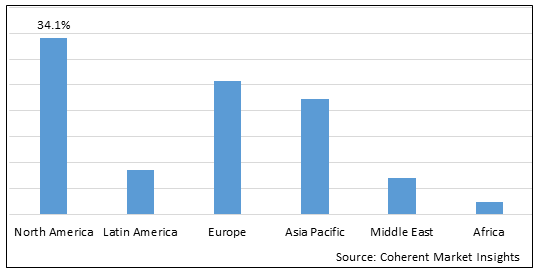
Figure 2. Global Chronic Liver Diseases Therapeutics Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Chronic Liver Diseases Therapeutics Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global chronic liver diseases therapeutics market over the forecast period. north america is estimated to hold 34.1% of the market share in 2025. The global chronic liver diseases therapeutics market is expected to witness significant growth in the coming years, driven by the high prevalence of cancer, favorable health reimbursement, and increased awareness, For instance, according to the press release in February 2020, by imaware, provides laboratory testing for wellness monitoring, informational, and educational use in North America, announced the launch of the At-Home Cancer Screening Test, designed by healthcare company Micro drop. The imaware test for cancer uses just a few drops of blood to detect unique biomarkers as well as two additional drugs to provide more comprehensive results. These kinds of developments in the healthcare system and rising health expenditures, as well as increased knowledge of advanced cancer treatments, are fueling the Chronic Liver Diseases Therapeutics market in North America.
Global Chronic Liver Diseases Therapeutics Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global Chronic Liver Diseases Therapeutics market. The COVID-19 outbreak affected the market's growth adversely in its preliminary phase; however, the number of cancer patients increased, and so has the demand for the chordoma disease market. For instance, according to an article published in February 2022 on PubMed Central, “COVID-19 and Cancer Cross talk: Emerging Association, Therapeutic Options and Challenges”, explains that the management of cancer patients in the COVID-19 context is a difficult task in and of itself. Even though online consultations are advisable with patients who are having stable cancer.
Chronic Liver Diseases Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 18.7 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.9% | 2032 Value Projection: | USD 38.58 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Astellas Pharma Inc., Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline Plc, F. Hoffmann-La Roche Ltd., Merck & Co. Inc., Novartis AG, Sanofi S.A, Pfizer Inc., Takeda Pharmaceutical, Valeant Pharmaceuticals, Watson Pharmaceuticals, Inc., Theratechnologies Inc., Alnylam Pharmaceuticals, Inc., Protagonist Therapeutics, Inc., Dicerna Pharmaceuticals, Inc., Endo International, Provectus Biopharmaceuticals Inc., MAX BioPharma, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Chronic Liver Diseases Therapeutics Market Segmentation:
The global chronic liver diseases therapeutics market report is segmented By Treatment Type, By Disease Type, By Distribution type, and By Region.
By Treatment Type, the market is segmented into Antiviral drugs, immunosuppressant’s, vaccines, immunoglobulin’s, corticosteroids, targeted therapy, and chemotherapy. Out of which, the antiviral drugs is expected to hold a dominant position in the global chronic liver diseases therapeutics market during the forecast period and this is attributed to awareness about liver diseases.
By Disease Type, the market is segmented into hepatitis, autoimmune diseases, non-alcoholic fatty liver disease (nafld), cancer, genetic disorders, and others. Out of which, the Hepatitis is expected to dominate the market over the forecast period and this is attributed to increasing heavy alcohol use, toxins, some medications, and certain medical conditions.
By Distribution Channel, the market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Out of which, Hospital pharmacies is expected to dominate the market over the forecast period and this is attributed to prevention, diagnosis, multidisciplinary treatment, supportive care, research and education.
Among all the segmentations, the treatment type segment expected to dominate the market over the forecast period and this is attributed to the block cancer growth in liver disease is which is expected boost the growth of chronic liver diseases therapeutics market over the forecast period.
Global Chronic Liver Diseases Therapeutics Market Cross Sectional Analysis:
Key players are making Chronic Liver Diseases Therapeutics with proper drug treatment emerging economies is also expected to boost demand chronic liver diseases therapeutics market in North America region. For instance, in October 2020, Myriad Genetic Laboratories, Inc., a genetic testing and precision medicine company based in Salt Lake City, Utah, U.S., introduced Myriad's EGFR (estimated glomerular filtration rate) inhibitor treatment method from the Japanese Ministry of Health, Labor, and Welfare to be utilized as a partnered treatment.
Global Chronic Liver Diseases Therapeutics Market: Key Developments
In September 2021, Astellas is a multinational pharmaceutical company in Japan, announced that it has voluntarily paused screening and dosing of additional participants in its ASPIRO clinical trial evaluating AT132 in patients with X-linked Myotubular Myopathy (XLMTM). This decision follows the reporting of a recent serious adverse event (SAE) in a study participant due to abnormal liver function tests (LFTs) observed in the weeks following dosing of the AT132 investigational gene therapy product at a lower dose (1.3x1014 vg/kg). Astellas voluntarily halted screening and dosing, reported the SAE to regulatory agencies, and is engaged in dialogue with regulators about this SAE.
On May 13, 2023, Astellas Pharma Inc., a multinational pharmaceutical company in Japan, announced that the U.S. Food and Drug Administration (FDA) has approved VEOZAHTM (fezolinetant) 45 mg once daily for the treatment of moderate to severe vasomotor symptoms (VMS) due to menopause1 on May 12. VEOZAH is the first non-hormonal neurokinin 3 (NK3) receptor antagonist approved to treat VMS due to menopause.
In October 2022, Bristol Myers Squibb, a biological and pharmaceutical research, producing the antipsychotic Abilify company, announced new results from the POETYK PSO long-term extension (LTE) trial demonstrating clinical efficacy was maintained with continuous Sotyktu (deucravacitinib) treatment in adult patients with moderate-to-severe plaque psoriasis. This analysis assessed patients from the pivotal POETYK PSO-1 trial transitioned into the LTE trial. At 112 weeks of Sotyktu treatment, modified non-responder imputation (mNRI) response rates were 82.4% for Psoriasis Area and Severity Index (PASI) 75, 55.2% for PASI 90 and 66.5% for static Physician's Global Assessment (sPGA) 0/1.
In June 2022, Gilead Sciences, Inc. is a research-based biopharmaceutical company focused on the discovery, development, and commercialization of innovative medicines, announced that more than 80 abstracts will be presented at the International Liver Congress (ILC) 2022, placed from June 22-26, 2022. Key oral presentations will include Week 48 primary endpoint data from the Pivotal Phase 3 program of Hepcludex (bulevirtide) evaluating its efficacy and safety for the treatment of hepatitis delta virus (HDV) and the impact of the treatment on patient-reported outcomes.
On June 1, 2023, Myriad Genetics, Inc., a company of genetic testing and precision medicine, launched new studies and expansion of its Precise Oncology Solutions portfolio at the 2023 American Society of Clinical Oncology (ASCO).
On March 22, 2023, F. Hoffmann-La Roche Ltd (Roche), a biotechnology company that develops drugs and diagnostics to treat major diseases, announced that it has entered into a collaboration with Eli Lilly and Company, a pharmaceutical company headquartered in U.S. to support the development of Roche’s Elecsys Chronic Liver Diseases Therapeutics.
In November 2022, GSK plc, a multinational pharmaceutical and biotechnology company with global headquarters in London, announced the publication of positive end of study results from the B-Clear phase IIb trial evaluating the safety and efficacy of bepirovirsen for the treatment of chronic hepatitis B (CHB) in the New England Journal of Medicine. The results showed that treatment with bepirovirsen, an investigational antisense oligonucleotide treatment, resulted in sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA both in patients on concurrent nucleoside/nucleotide analogues (NA) and patients not-on-NA therapy.
In June 2022, GSK plc, a multinational pharmaceutical and biotechnology company with global headquarters in London, announced promising interim results from the B-Clear phase IIb trial showing that bepirovirsen, an investigational antisense oligonucleotide treatment for hepatitis B, reduced levels of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA after 24 weeks’ treatment in people with chronic hepatitis B (CHB).
In March 2022, Invitae, a medical genetics company, announced full access to its Personalized Cancer Monitoring (PCMTM) platform to help detect minimal or molecular residual disease (MRD) in patients with solid tumors. Invitae PCM uses a novel set of personalized assays based on a patient's tumor to detect circulating tumor DNA (ctDNA) in blood, offering the ability to perform risk stratification, response assessment to treatment and detection of cancer recurrence, based on recent studies.In November 2022, Arrowhead Pharmaceuticals Inc., develops medicines that treat intractable diseases by silencing the genes, entered a drug development deal with GlaxoSmithKline Plc (GSK.L), a multinational pharmaceutical and biotechnology company with global headquarters in London, under which the British drugmaker will develop and market Arrowhead's potential treatment for patients with fatty liver disease NASH.
Global Chronic Liver Diseases Therapeutics Market: Key Trends
Application of biomarker test for diagnosing liver disease
Application of biomarker test for diagnosing liver disease is expected to propel growth of the global chronic liver diseases therapeutics market over the forecast period. Major players in the market are focused on adopting partnership strategies to expand its product portfolio. Presently, there is no drugs available for the diagnosis of chordoma disease. However, anti–cyclic citrullinated peptide (anti–CCP) antibodies test require more refinement to improve its clinical utility. For instance, in March 2020, WorldCare Clinical, LLC, an independent contract research organization offering imaging in clinical trials, partnered with Navidea Biopharmaceuticals, Inc., a biopharmaceutical company focused on the development of precision immunodiagnostic agents and immunotherapeutics, to offer imaging service following U.S. FDA approval of Navidea’s cancer diagnostic. Navidea’s strategy is to deliver novel products and advancing the company’s pipeline through global partnering with WorldCare Clinical, LLC.
Global Chronic Liver Diseases Therapeutics Market: Restraint
Chemotherapeutic drugs approved for the treatment of liver are showing side effects
Chemotherapeutic drugs approved for the treatment of liver are not always effective and have several side effects such as nausea, headache, and hair loss is expected to hamper the growth of the global chronic liver diseases therapeutics market. For instance, according to an article published in the National library of medicine, on February 27, 2023, reported antimetabolites have a several side effects such as dose-limiting myelosuppression. Toxic levels of 5-fluorouracil occur in patients with Dihydropyrimidine Dehydrogenase (DPD) deficiency or drug overdose. This lead to cardiac dysfunction, colitis, neutropenia, and encephalopathy.
Key market players are focusing on safety and efficacy of drugs.
Global Chronic Liver Diseases Therapeutics Market- Key Players
Major players operating in the global Chronic Liver Diseases Therapeutics market include Astellas Pharma Inc., Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline Plc, F. Hoffmann-La Roche Ltd., Merck & Co. Inc., Novartis AG, Sanofi S.A, Pfizer Inc., Takeda Pharmaceutical, Valeant Pharmaceuticals, Watson Pharmaceuticals, Inc., Theratechnologies Inc., Alnylam Pharmaceuticals, Inc., Protagonist Therapeutics, Inc., Dicerna Pharmaceuticals, Inc., Endo International, Provectus Biopharmaceuticals Inc., and MAX BioPharma, Inc.
*Definition: Chronic liver disease is a gradual deterioration of liver functions. Liver function comprises the excretion of bile, detoxification of harmful products of metabolism, production of clotting factors, and other proteins. Chronic liver disease is a constant process of destruction, inflammation, and regeneration of the liver parenchyma, which leads to fibrosis and cirrhosis. The causes of chronic liver diseases include alcohol abuse, toxins, infection, autoimmune diseases, genetic and metabolic disorders.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients