Global chronic kidney disease drugs market is estimated to be valued at USD 15.92 Billion in 2025 and is expected to reach USD 23.02 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 5.4% from 2025 to 2032. Increasing geriatric population suffering from chronic conditions such as diabetes and hypertension, rising awareness about early diagnosis, and growing demand for drugs to slow the progression of CKD can drive the market growth.
Discover market dynamics shaping the industry: Download Free Sample
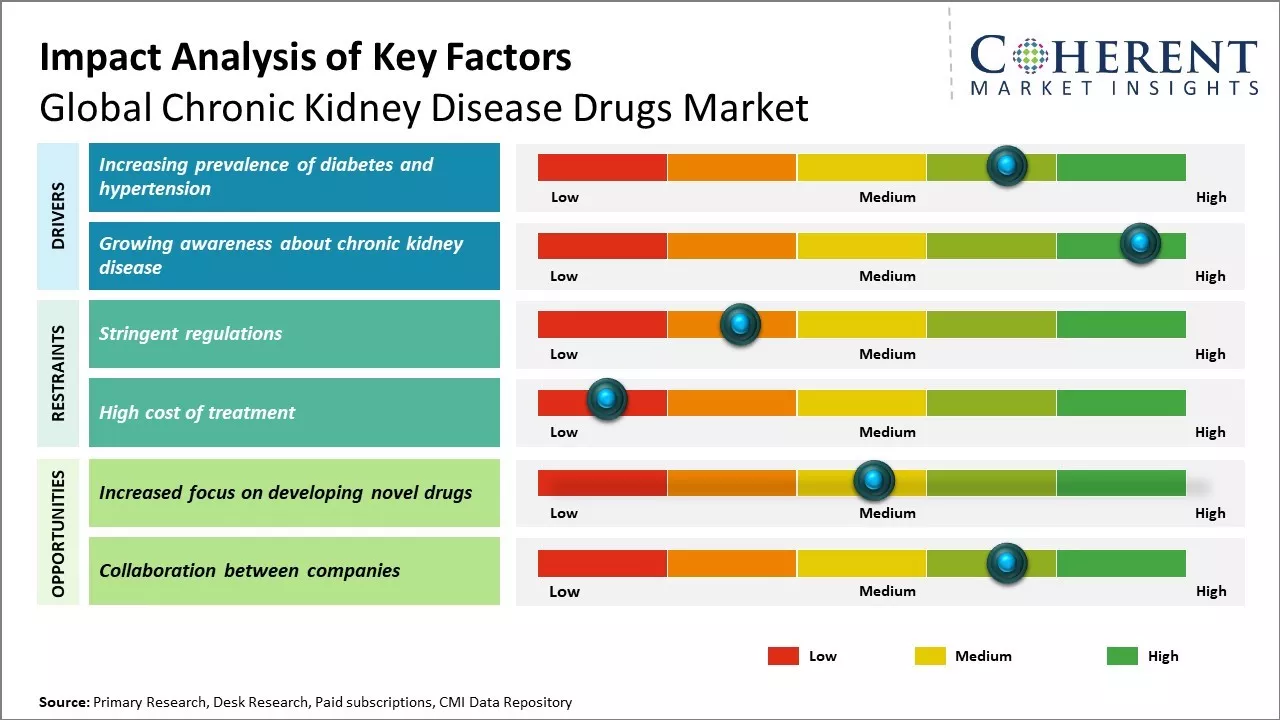
Market Driver - Increasing prevalence of diabetes and hypertension
Rising prevalence of diabetes and hypertension across major regions of the world can drive the chronic kidney disease drugs market growth. Diabetes and hypertension are among the leading causes of chronic kidney disease as these can damage the kidneys over a prolonged period of time, if not managed properly. According to studies, almost 40% of patients with diabetes and 30% of those with hypertension will eventually develop some form of chronic kidney disease. With diabetes rates rising due to growing obesity, sedentary lifestyles and aging populations, there has been corresponding increase in chronic kidney disease cases linked to diabetes. Statistics showed that countries with high diabetes prevalence like the U.S., China, India, Brazil, Mexico, and others have witnessed fastest growth in chronic kidney disease cases attributed to uncontrolled diabetes. Even regions like the Middle East and Africa that already had a high burden of diabetes and hypertension witnessed a surge in end-stage renal disease. This compels healthcare systems to spend more resources on kidney disease treatment and dialysis, placing immense pressure. With no immediately foreseeable decline in the incidence of diabetes and hypertension globally, there will be rise in prevalence of chronic kidney disease caused by these conditions. This growing patient pool suffering from kidney ailments can boost demand for chronic kidney disease drugs for long-term therapy and management.
Get actionable strategies to beat competition: Download Free Sample
Growing awareness about chronic kidney disease
In the past, chronic kidney disease often went undetected in its early stages due to lack of symptoms and awareness among at-risk groups as well as physicians. Thus, many patients reached end-stage renal disease requiring expensive dialysis or transplant. However, over time awareness campaigns by government agencies, healthcare non-profits as well as pharmaceutical companies have led to heightened screening and diagnosis of chronic kidney disease. Nowadays, more population groups understand their risk of kidney ailments depending on age, family history of kidney disease, pre-existing conditions like diabetes or high blood pressure. Even general physicians are more proactive in testing kidney function routinely as part of general health checkups for high-risk individuals.
This helps to detect chronic kidney disease much earlier when treatment and therapeutic interventions can slow progression and effectively manage the ailment. Early detection also enhances focus on lifestyle modifications, diet control and medication adherence which are imperative in renal care. With growing cognizance, patients are preferring long-term therapeutic options over temporary treatments like dialysis. All these factors have prompted increased uptake of chronic kidney disease drugs that offer long-lasting solutions from the initial stages itself. Pharma companies are aggressively creating awareness through disease education initiatives, screening campaigns as well as new product developments to cater to evolving patient needs. As awareness proliferation continues across both patients and healthcare community, it will boost demand for chronic kidney disease pharmacological interventions.
Key Takeaways from Analyst:
Global chronic kidney disease drugs market growth is driven by rising prevalence of diabetes, hypertension, and other kidney-related diseases. Growing geriatric population who are more prone to developing chronic kidney issues can also drive the market growth. North America currently dominates the market, owing to high healthcare expenditures and availability of advanced treatment options. However, Asia Pacific is likely to emerge as the fastest growing regional market in the near future.
High costs associated with kidney disease treatments can hamper the market growth, especially in price-sensitive developing regions. Lack of kidney donors for transplant procedures can also hamper the market growth. However, ongoing pipeline of new drug candidates offers lucrative opportunities for manufacturers. Increasing R&D investments by key market players to develop novel drugs with improved efficacy and reduced side effects can drive the market growth. Partnerships with healthcare providers to spread awareness about early detection and management of chronic kidney diseases can also offer growth opportunities.
Market Challenge - Stringent regulations
Global chronic kidney disease drugs market growth can be hampered by stringent regulations posed by regulatory authorities for approval and launch of new drugs. Drug development and approval process is a complex, time-consuming, and costly affair. Regulatory bodies such as U.S. FDA and EMA extensively evaluate clinical trial data for safety and efficacy before approving a drug for commercialization. Any non-compliance during drug development or lapses in post marketing surveillance can attract penalties and revocation of approvals. Moreover, regulations are frequently updated, necessitating modifications in manufacturing and operations. These stringent norms increase uncertainty and financial risk for pharmaceutical companies. Long review periods result in delay in treating patients and loss of revenue, owing to delayed market entry of products. The industry has to spend significantly on ensuring compliance to dynamic regulatory frameworks.
Market Opportunities-Novel Drugs
The focus on developing novel drugs for chronic kidney disease presents lucrative opportunities for players in the market. There is high unmet need for more effective and specialized therapeutics with improved safety profiles. Rising research and development investments by leading producers in studying new molecular entities and targets can lead to launch of breakthrough medications. Various biologics and targeted oral therapies are under clinical trials aiming to slow or halt progression of kidney damage. The success of these pipeline drugs can drive the market growth in the near future. Furthermore, acquisitions and partnerships between companies and research institutions to expand product pipelines will strengthen market position and foster innovation.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
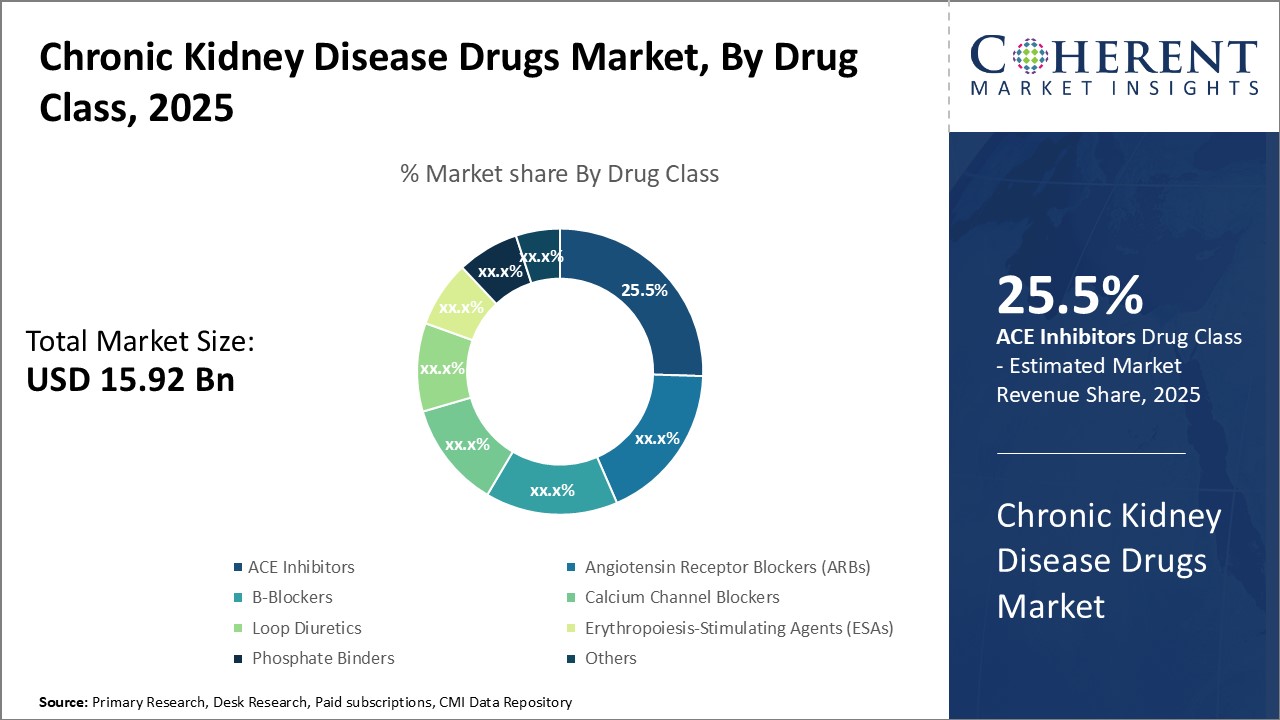
By Drug Class- Increasing prevalence of chronic kidney diseases drives growth of ACE inhibitors
In terms of drug class, ACE inhibitors segment is estimated to contribute the highest market share of 25.5% in 2025. ACE inhibitors are generally the first line of treatment recommended for various chronic kidney diseases like diabetic nephropathy and hypertensive nephropathy due to their effectiveness in slowing progression of kidney damage by lowering blood pressure and reducing proteinuria. Their long-term use is well established and proven benefits have entrenched them as standard of care treatment for renal conditions. Growing prevalence of diabetes and hypertension fueled by lifestyle changes and risk factors has substantially increased incidence of chronic kidney diseases globally. Rising prevalence of these conditions leads to high use of ACE inhibitors, thus, burgeoning cases of associated renal disorders can boost demand for ACE inhibitors. Innovative drug delivery formulations launched by major players have enhanced medication adherence and made ACE inhibitors more convenient for chronic kidney disease patients to manage their conditions.
By Route of Administration- Convenience drives oral segment growth
In terms of route of administration, oral segment is estimated to contribute the highest market share of 62.5% in 2025, owing to convenience and ease of use advantages over parenteral drugs. High efficacy demonstrated by oral medications over long term has established them as first line treatment choice for chronic kidney diseases. Being non-invasive in nature, oral drugs are generally preferred by patients and clinicians alike for chronic therapy adherence. Self-administration of pills also saves resources by reducing hospital visits for injections. This has led to extensive research and development of novel drug delivery systems like timed and controlled release formulations, thus, enhancing oral bioavailability and stability of drugs. Moreover, ability to take medications conveniently without medical supervision has powered compliant oral administration across care settings for chronic kidney disease patients.
By Indication- Rising incidence of diabetic nephropathy drives the segment growth
In terms of indication, diabetic nephropathy segment is estimated to contribute the highest market share of 46.5% in 2025, due to alarming increase in incidence rates due to diabetes epidemic. Diabetic nephropathy is one of the most common causes of chronic kidney diseases associated with poor glycemic control that can damage kidneys. With almost half a billion adults currently living with diabetes worldwide and projections of significant rise, cases of resulting kidney complications have skyrocketed. Early detection and optimized treatment strategies have failed to curb its progression entirely. Thus, sustained demand for therapeutic drugs from expanded diabetic nephropathy patient pool can drive the segment growth. Furthermore, limited treatment alternatives also account for high dependence on available drugs.
Need a Different Region or Segment? Download Free Sample
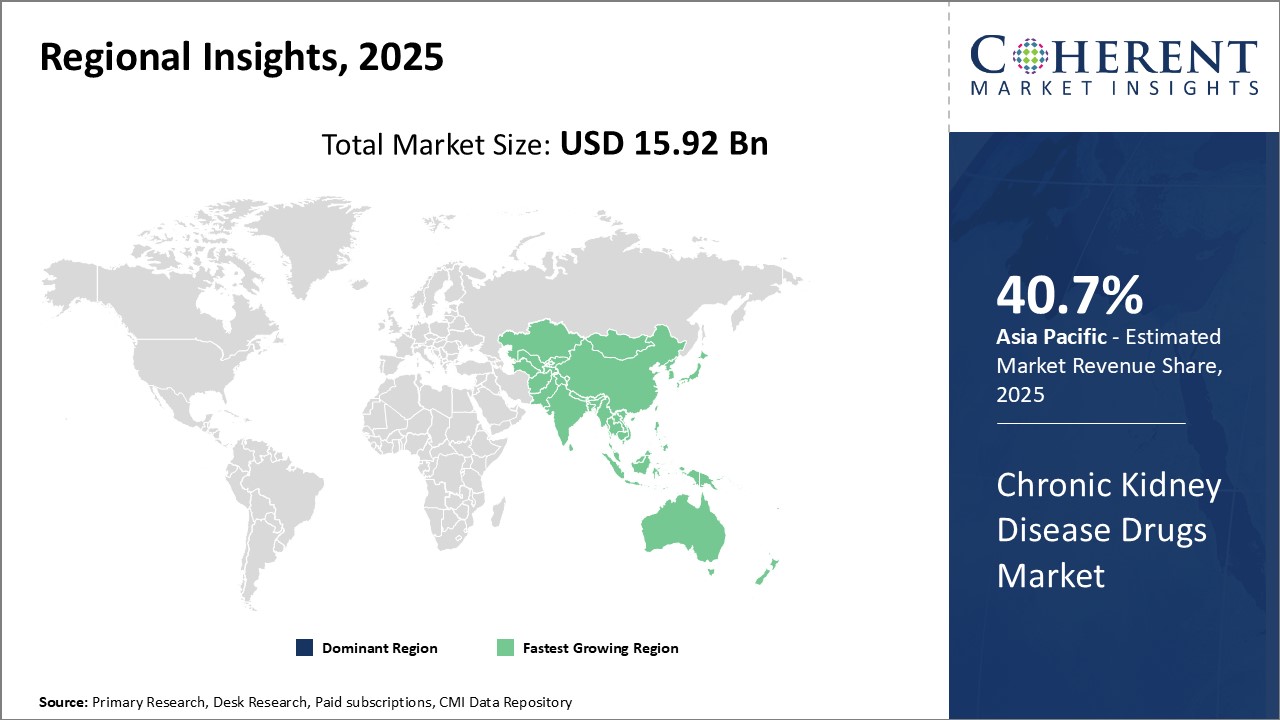
North America is expected to dominate the global chronic kidney disease drugs market with an estimated market share of 40.7% in 2025. The U.S. accounts for the major share due to the high prevalence of chronic kidney diseases in the country. As per studies, over 30 million Americans suffered from chronic kidney diseases in 2020. The healthcare infrastructure and spending are among the best in the world which has led to better availability of advanced chronic kidney disease drugs in this region.
Moreover, major pharmaceutical companies have their presence in theU.S. These allocate large investments in R&D activities for novel drug development. This has resulted in the early availability and adoption of newer treatment options. Reimbursement programs like Medicare provide insurance coverage for chronic kidney disease drugs as well which facilitates higher affordability for patients. Asia Pacific region has emerged as the fastest growing market. China and India are spearheading this growth due to rising healthcare investments, improving access to healthcare facilities, and increasing healthcare awareness. The rapidly increasing geriatric population who are vulnerable to chronic diseases like diabetes and hypertension has boosted the cases of chronic kidney diseases in these nations.
This has attracted pharmaceutical giants to focus on Asia Pacific through joint ventures with local players or establishment of manufacturing units. These promote awareness and education programs about chronic kidney diseases and treatments which has boosted demand. Favorable government initiatives to make medicines affordable further drives the market growth. Growing economies, large patient pool, and supportive regulatory environment make Asia Pacific an attractive market for chronic kidney disease drugs.
Chronic Kidney Disease Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 15.92 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.4% | 2032 Value Projection: | USD 23.02 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
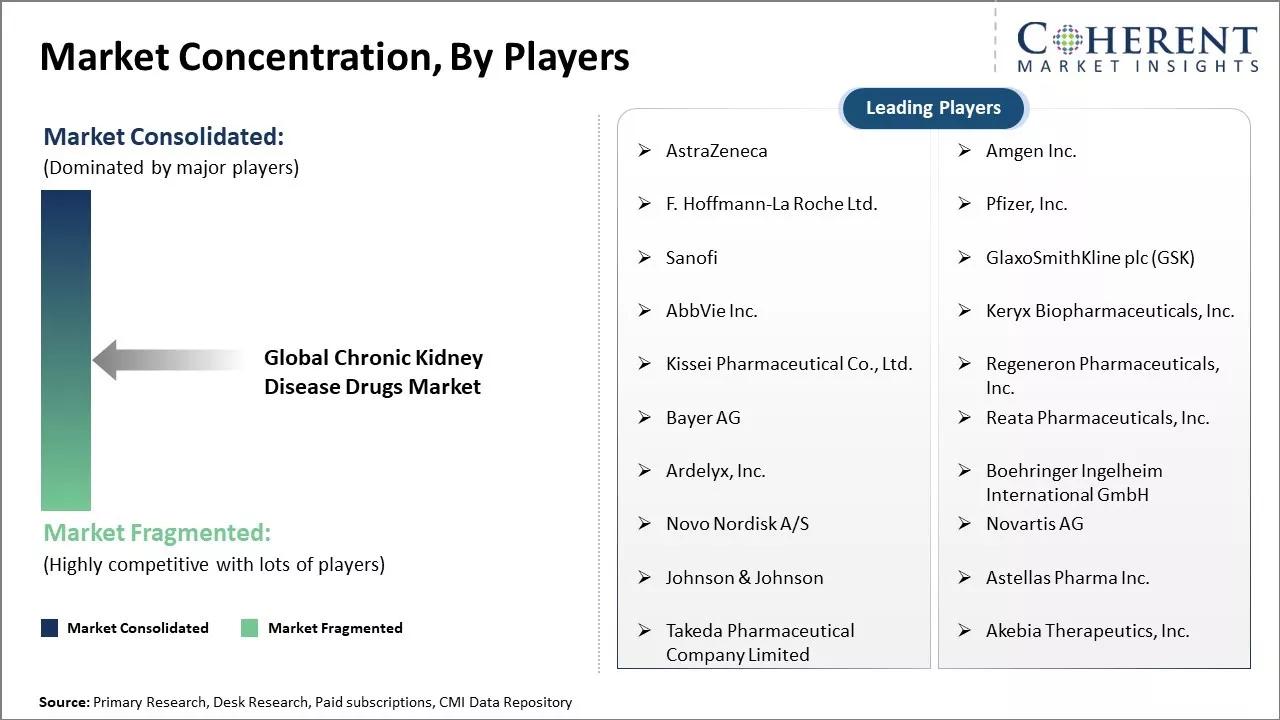
| Companies covered: |
AstraZeneca, Amgen Inc., F. Hoffmann-La Roche Ltd., Pfizer, Inc., Sanofi, GlaxoSmithKline plc (GSK), AbbVie Inc., Keryx Biopharmaceuticals, Inc., Kissei Pharmaceutical Co., Ltd., Regeneron Pharmaceuticals, Inc., Bayer AG, Reata Pharmaceuticals, Inc., Ardelyx, Inc., Boehringer Ingelheim International GmbH, Novo Nordisk A/S, Novartis AG, Johnson & Johnson, Astellas Pharma Inc., Takeda Pharmaceutical Company Limited, Akebia Therapeutics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global chronic kidney disease drugs market includes pharmaceutical products used to manage chronic kidney disease. Key drug classes in this market are ACE inhibitors, calcium channel blockers, beta-blockers, erythropoiesis-stimulating agents (ESAs), phosphate binders, vitamin D sterols, and diuretics. These medications are prescribed to slow kidney damage progression, manage anemia, lower blood pressure, control electrolyte levels such as phosphorus, and address associated complications.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients