Cholangiocarcinoma Market is estimated to be valued at USD 618.3 Mn in 2025 and is expected to reach USD 2,334.4 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 20.9% from 2025 to 2032.
Analysts’ Views on the Global Cholangiocarcinoma Market:
Bile ducts are tiny canals that connect some of the digestive system's organs such as liver and gallbladder. Their function is to transport bile between these organs. The biliary system is made up of organs and bile ducts. It consists of the liver, gallbladder, and small intestine. Cholangiocarcinoma is a cancer that develops in the bile ducts. It is a rare yet aggressive form of cancer. Yellow skin and eyes (jaundice), intensely itchy skin, and white stool are some of the symptoms of cholangiocarcinoma. Product approvals of innovative drugs are anticipated to drive the market development over the forecast period. For instance, in September 2022, Taiho Oncology, Inc., a subsidiary of Taiho Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd., a subsidiary of Otsuka Holdings Co., Ltd., announced that the U.S. Food and Drug Administration (FDA) had approved LYTGOBI tablets for the treatment of adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma (iCCA), harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.
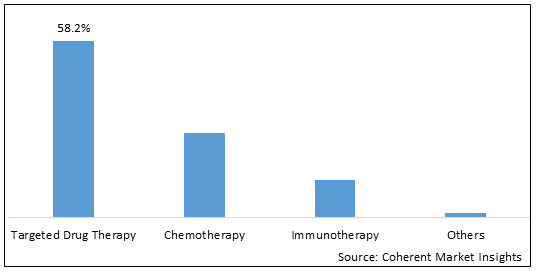
Figure 1. Global Cholangiocarcinoma Market Share (%), By Therapy Type, 2025
To learn more about this report, Download Free Sample
Global Cholangiocarcinoma Market - Drivers
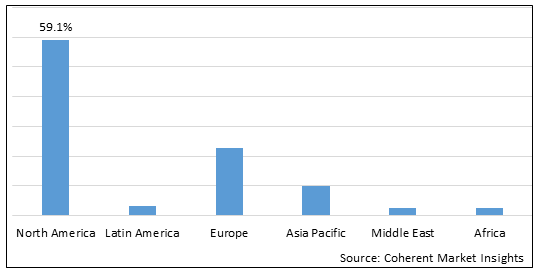
Figure 2. Global Cholangiocarcinoma Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Cholangiocarcinoma Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global cholangiocarcinoma market over the forecast period. North America is estimated to hold 59.1% of the market share in 2025. North America cholangiocarcinoma market is expected to witness significant growth in the near future, owing to approval of new drugs in the market. For instance, in May 2021, the U.S. FDA granted accelerated approval to infigratinib (Truseltiq, QED Therapeutics, Inc., a biotechnology company), a kinase inhibitor for adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test.
Cholangiocarcinoma Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 618.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 20.9% | 2032 Value Projection: | USD 2,334.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
BridgeBio Inc., Sanofi, Eisai Co., Ltd., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Intas Pharmaceuticals Ltd., Fresenius SE & Co. KGaA, Sun Pharmaceutical Industries Ltd., Hikma Pharmaceuticals PLC, Incyte, TRISALUS LIFE SCIENCES, INC., GENFIT, Johnson & Johnson Private Limited, Bliss Biopharmaceutical, Novartis AG, LES LABORATOIRES SERVIER, F. Hoffmann-La Roche Ltd, Mylan N.V. (Viatris.Inc), AstraZeneca, Compass Therapeutics, Inc, Otsuka Holdings Co., Ltd., and Senhwa Biosciences, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Cholangiocarcinoma Market - Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
The supply of raw materials required for the production of drugs intended for the treatment of cholangiocarcinoma was severely disrupted due to forced quarantine measures and lack of labor during the COVID-19 pandemic. Owing to disruptions in the link between regional warehouses, the transportation of raw materials between regions could not be carried out successfully. This shortage of raw materials and components hampered the supply chain of the global cholangiocarcinoma market.
Due to the unprecedented burden on healthcare resources, many healthcare activities such as chronic disease management, cancer screening, and cancer treatments were cancelled or delayed. Consequently, referrals of suspected new cancers have reduced, with increase in cancer-related deaths to be estimated during the pandemic period. According to data published by National Center for Biotechnology Information (NCBI), in July 2021, 63% of patients in Saudi Arabia reported a delay in treatment during the COVID-19 pandemic. The key reason behind this delay was hospital cancellations or procedure delays. A third of patients (30.8%) reported lack of cancer support and shortage of medications during the pandemic. Almost all were fearful of contracting the virus in healthcare settings and over 80% experienced an adverse impact on quality of life in 2020.
Global Cholangiocarcinoma Market- Segmentation
Global cholangiocarcinoma market is segmented based on cancer type, therapy type, route of administration, distribution channel, and region.
Among all the segments, the immunotherapy segment is expected to develop at the highest CAGR over the forecast period, owing to the large number of ongoing clinical studies to study the efficacy of novel drugs to treat cholangiocarcinoma.
Global Cholangiocarcinoma Market - Cross Sectional Analysis
An increase in the adoption of inorganic growth strategies such as acquisition by the key players in Asia Pacific region is expected to drive the chemotherapy segment growth over the forecast period. For instance, on January 23, 2023, CHEPLAPHARM, a pharmaceutical company, announced the acquisition of the commercial rights for Xeloda (capecitabine) in China from F. Hoffmann-La Roche Ltd, a global pharmaceutical company. Capecitabine is a chemotherapy drug used for the treatment of cholangiocarcinoma. CHEPLAPHARM strengthens its cancer portfolio and expands its foothold in the world's second largest pharmaceutical market by adding China.
Global Cholangiocarcinoma Market- Key Developments
Global Cholangiocarcinoma Market: Key Trends
Global Cholangiocarcinoma Market: Restraint
Global Cholangiocarcinoma Market - Key Players
The major players operating in the global cholangiocarcinoma market include BridgeBio Inc., Sanofi, Eisai Co., Ltd., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Intas Pharmaceuticals Ltd., Fresenius SE & Co. KGaA, Sun Pharmaceutical Industries Ltd., Hikma Pharmaceuticals PLC, Incyte, TRISALUS LIFE SCIENCES, INC., GENFIT, Johnson & Johnson Private Limited, Blis Biopharmaceutical, Novartis AG, LES LABORATOIRES SERVIER, F. Hoffmann-La Roche Ltd, Mylan N.V. (Viatris.Inc), AstraZeneca, Compass Therapeutics, Inc, Otsuka Holdings Co., Ltd., and Senhwa Biosciences, Inc.
Definition: Cholangiocarcinoma is a type of cancer that forms in the slender tubes (bile ducts) which carries the digestive fluid bile. The specific reason for the cause of cholangiocarcinoma is still unknown. The malignant tumor may arise from any portion of the bile duct i.e., from terminal ductules (canals of Hering) to the ampulla of Vater, also at the peribiliary glands (intramural and extramural) which may sometimes affect the gall bladder as well.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients