Mesalazine is an aminosalicylate drug used to treat mild to moderate active ulcerative colitis and maintain remission once achieved. Mesalazine is an anti-inflammatory agent, structurally related to salicylates and non-steroidal anti-inflammatory drugs like acetylsalicylic acid, which is used in the treatment of inflammatory bowel diseases like ulcerative colitis and Crohn’s disease. Ulcerative colitis is a type of chronic inflammatory bowel disease that causes inflammation of the large intestine lining (colon). It produces ulcers on the colon's lining, which may cause bleeding and discharge of pus and mucus. Mesalazine works by inhibiting the production of certain chemical substances such as prostaglandins that cause pain and swelling that helps in reducing inflammation (redness and swelling) in the intestines and provides relief from symptoms such as stomach pain or bleeding.
China mesalazine market is estimated to be valued at US$ 51.6 million in 2022 and is expected to exhibit a CAGR of 3.1% during the forecast period (2022-2030).
Figure 1. China Mesalazine Market Share (%), by Route of Administration, 2022
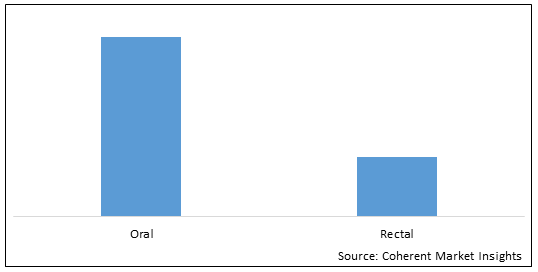
To learn more about this report, Download Free Sample
China Mesalazine Market - Driver
Increasing inorganic strategies, such as product launches by key market players, is expected to drive the growth of China mesalazine market. For instance, in July 2021, Tillotts Pharma AG, a pharmaceutical company, launched Asacol 800 mg tablets (mesalazine) as the first-line treatment for mild to moderate Ulcerative Colitis (UC) in China. It will strength mesalazine tablet formulation to the Chinese market to optimize the therapeutic options of patients living with ulcerative colitis in China.
China Mesalazine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 51.6 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 3.1% | 2030 Value Projection: | US$ 65.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Zhejiang Hengkang Pharmaceutical Co. Ltd., Jiangsu Jubang Pharmaceutical Co., Ltd., ALP Pharm Beijing Co., Ltd., Shanghai Pharmaceuticals Holding Co Ltd., Heilongjiang Tianhong Pharmaceutical Co., Ltd., AbbVie Inc., F. Hoffmann-La Roche AG, Takeda Pharmaceuticals Company Limited, GSK Plc., Tillotts Pharma AG, Novartis AG, Salix Pharmaceuticals, FERRING B.V., Viatris Inc., and Dr. Falk Pharma GmbH |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. China Mesalazine Market Share (%), by Application, 2022
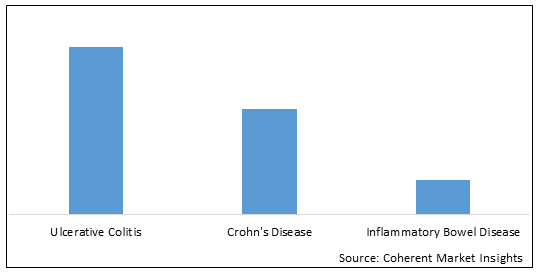
To learn more about this report, Download Free Sample
China Mesalazine Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
The COVID-19 can affect the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on China mesalazine market. For instance, according to the data published in May 2020 by the National Center for Biotechnology Information, the COVID-19 pandemic affected patients with Inflammatory Bowel Disease (IBD) medically and psychosocially. Most patients with IBD experienced disease during the early and current phases of the outbreak and those individuals did not have to change their medications.
China Mesalazine Market: Key Developments
Market players are engaged in joint ventures which is expected to drive China mesalazine market growth over the forecast period. For instance, in November 2022, Alembic Pharmaceutical Limited, a pharmaceutical company, announced that it had received approval from the U.S. Food and Drug Administration (U.S. FDA) for the Abbreviated New Drug Application (ANDA) for Mesalazine extended–release capsules of strength 0.375 g.
China Mesalazine Market: Restraint
The availability of alternatives for mesalazine is expected to hamper the growth of China mesalazine market over the forecast period. Aminosalicylate (5-ASA) is considered the first line of treatment for ulcerative colitis. Moreover, other drug classes are indicated for the treatment of ulcerative colitis, inflammatory bowel disease, and Crohn’s disease. They are Corticosteroids, Immunomodulators, Targeted Synthetic Small Molecules (Immunosuppressants), and Biologic/Biosimilar Therapies (Monoclonal Antibodies). Furthermore, the introduction of biosimilars has revolutionized the control of Ulcerative Colitis (UC). It is a biological treatment for inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease. These medicines are less expensive than other drugs, making them more accessible to people in China.
China Mesalazine Market - Key Players
Major players operating in China mesalazine market include Zhejiang Hengkang Pharmaceutical Co. Ltd., Jiangsu Jubang Pharmaceutical Co., Ltd., ALP Pharm Beijing Co., Ltd., Shanghai Pharmaceuticals Holding Co Ltd., Heilongjiang Tianhong Pharmaceutical Co., Ltd., AbbVie Inc., F. Hoffmann-La Roche AG, Takeda Pharmaceuticals Company Limited, GSK Plc., Tillotts Pharma AG, Novartis AG, Salix Pharmaceuticals, FERRING B.V., Viatris Inc., and Dr. Falk Pharma GmbH.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients