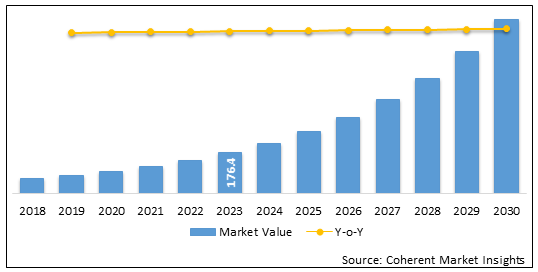
China Continuous Glucose Monitoring Devices Market is estimated to be valued at USD 265.4 Mn in 2025 and is expected to reach USD 1,117.6 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 22.8% from 2025 to 2032.
Analysts’ Views on China Continuous Glucose Monitoring Devices Market :
The increase in geriatric population and prevalence of diabetes in China is expected to drive the growth of the China continuous glucose monitoring devices market over the forecast period. For instance, according to an article published by The Lancet Regional Health, on February 3, 2025, diabetes prevalence in Chinese adults aged 20–79 years was projected to increase from 8.2% to 9.7% during 2020–2032. During the same period, the total costs of diabetes would increase from $250.2 billion to $460.4 billion, corresponding to an annual growth rate of 6.32%.
Figure 1. China Continuous Glucose Monitoring Devices Market Value (US$ Mn) & Y-o-Y Growth (%), 2020-2032
To learn more about this report, Download Free Sample
China Continuous Glucose Monitoring Devices Market – Driver
Increasing collaborations of key market players
Increasing collaboration between key market players is expected to propel the growth of China continuous glucose monitoring devices market over the forecast period. For instance, on January 23, 2020, DKSH Holding Ltd., a Switzerland-based healthcare solution company, entered into collaboration with LifeScan IP Holdings, LLC., a U.S.-based diagnostic system manufacturer. Under the agreement, DKSH will provide marketing, sales, and regulatory services as well as distribution and logistics for LifeScan across Asian markets.
Launch of new products in market
Increasing launches of new products are also expected to aid in the growth of the market. For instance, on April 14, 2023, Abbott Laboratories, U.S. based medical device company launched its FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system, which is a small handheld device that displays real-time glucose readings directly from a small sensor worn on the back of a person's upper arm, allowing them to manage their diabetes quickly and easily by viewing their glucose readings on a large, bright and easy-to-see screen.
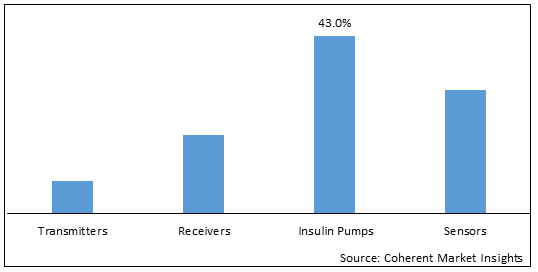
Figure 2. China Continuous Glucose Monitoring Devices Market Share (%), by Component, 2025
To learn more about this report, Download Free Sample
China Continuous Glucose Monitoring Devices Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the manufacturing and distribution of products due to lockdown, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Also due to social distancing, the procedures were reduced. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
The COVID-19 pandemic had a positive impact on the China continuous glucose monitoring devices market. This is due to the patients already having diabetes being at higher risk of COVID-19. According to data shared by China CDC on April 3, 2020, the case-fatality rate (CFR) for COVID-19 in China is more elevated among patients with pre-existing conditions from non-communicable disease (NCDs) - 10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer. Heart disease and stroke, cancer, chronic lung disease, and diabetes already cause 88% of deaths in China. Now, a person suffering from any of these NCDs is more likely to be fatal if they catch COVID-19.
China Continuous Glucose Monitoring Devices Market Segmentation:
The China continuous glucose monitoring devices market report is segmented into component and end user.
Based on Component, the market is segmented into transmitters, receivers, insulin pumps, and sensors. Out of which, the insulin pump segment is expected to hold a dominant position in the China continuous glucose monitoring devices market during the forecast period and this is attributed to launches of new products.
Based on End user , the market is segmented into hospital & clinics, homecare settings, and others. Out of which, hospital & clinics is expected to hold a dominant position in China continuous glucose monitoring devices market during the forecast period and this is attributed to the increasing number of hospitals.
Among all the segmentations, the component segment has the highest potential due to the increasing prevalence of insulin pumps over the forecast period. Increasing research and development activities to introduce newer insulin pumps with better efficacy contribute to growth of this segment. For instance, according to article published by Annals of translational medicine on November 30, 2020, efficacy and safety study of LenoMed ATA-I-1-0 insulin pump was carried out. It is a product of LenoMed, China-based medical equipment company. The insulin pump was compared with Medtronic, U.S. based medical technology company’s MMT-712 insulin pump. It was concluded that LenoMed ATA-I-1-0 insulin pump demonstrated efficacy and a good safety profile, and was not inferior to the widely used Medtronic MMT-712 insulin pump.
China Continuous Glucose Monitoring Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 265.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 22.8% | 2032 Value Projection: | USD 1,117.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic PLC, Abbott Laboratories, Medtrum Technologies, Inc., Senseonics, Nemaura, STMicroelectronics, NXP Semiconductors, Qualcomm, Taiwan Semiconductor Manufacturing Company Limited, GE Healthcare, Microchip Technology Inc., Texas Instruments Inc., Micron Technology Inc., Renesas Electronics Corporation, and Toshiba Corporation. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
China Continuous Glucose Monitoring Devices Market: Key Developments
Major players in the market are focused on research and development activities to expand their product portfolio. For instance, according to an article published by the journal Biosensors and Bioelectronics on February 6, 2021, a study was carried out to integrate interstitial fluid extraction and glucose detection in one device for wearable non-invasive blood glucose sensors. The feasibility of these non-invasive glucose sensors was validated on porcine skin, nude mice, and humans. The blood glucose concentration calculated according to the response currents of the two-electrode sensors is consistent with that measured by the commercial glucose meter. Furthermore, the used textile-like electrodes provide non-invasive blood glucose sensors with excellent flexible and wearable properties, which make them promising to be integrated with other electronic units for monitor and management of human health.
On September 26, 2021, Nordic Semiconductor, Europe based semiconductor company, announced that SiBionics Shenzhen, a China-based healthcare technology developer, has selected Nordic’s nRF52832 Bluetooth Low Energy (Bluetooth LE) general purpose multiprotocol System-on-Chip (SoC) to provide the core processing power and wireless connectivity for its ‘GS1 Continuous Glucose Monitoring (CGM) System’. GS1 CGM System is designed to enable patients to continuously monitor and record their interstitial fluid glucose levels in real-time for up to fourteen days before replacement. The system automatically generates an ambulatory glucose profile (AGP) for reference during medical consultation, and it won’t affect the patient’s everyday activities such as swimming or exercise.
On March 22, 2022, WaveForm Technologies,Inc. U.S. based diabetes equipment supplier having a manufacturing facility in China, announced it received CE mark approval for two important product improvements to the WaveForm Cascade Continuous Glucose Monitoring (CGM) system. It can be now worn on the upper arm. In addition to the new sensor site, improvements to the sensor algorithm have reduced the number of calibrations required for the Cascade system to one every two days.
On August 3, 2022, Actxa Pte. Ltd, a Singapore based digital health company, and Joint Chinese Limited, a China-based manufacturer of smart healthcare devices signed a Memorandum of Understanding (“MOU”), to join hands in market penetration and expansion for Pre-M DiabetesTM, also known as the Non-Invasive Blood Glucose Monitoring technology.
China Continuous Glucose Monitoring Devices Market : Key Trends
Approval of newer products and accessories
Product approval from regulatory bodies to newer glucose monitoring device accessories can drive growth of china continuous glucose monitoring devices market.
For instance, on November 7, 2022, Eli Lilly and Company, U.S. based global pharmaceutical company, launched the Tempo Personalized Diabetes Management, Platform which supports diabetes self-management through medication reminders, education resources, and insulin dose logging. Data shared through the platform interface may help healthcare providers to make data-driven decisions about care for adults treated with select Lilly insulin. This platform is compatible with Dexcom Continuous Glucose Monitoring System.
On June 24, 2021, Abbott Laboratories, U.S. based multinational medical device company collaborated with Beijing Medical Award Foundation to launch a demonstrative glucose monitoring program for more intelligent diabetes management in China. The program, namely the 2021 Continuous Glucose Monitoring Center of Excellence Program, aims to promote the application of a standardized continuous glucose monitoring system in hospitals across China. The first batch of hospitals, including Tianjin Medical University Chu Hsien-I Memorial Hospital and Hospital of Anhui Medical University, were recognized as the national demonstrative continuous glucose monitoring centers. The program is set to cover hundreds of hospitals across China in three years.
China Continuous Glucose Monitoring Devices Market: Restraint
Patient discomfort due to continuous glucose monitoring devices
Even though continuous glucose monitoring devices can reduce pain in customers with their minimally invasive technique, several discomforts like information overload and skin irritation can restrain the growth of the market. For instance, according to an article published by the Association Of Diabetes Care And Education Specialists on July 23, 2020, potential disadvantages with continuous glucose monitoring devices are that it may require calibration with fingerstick glucose, need to remember to scan an intermittently-scanned device it can be complicated to learn for elderly patients and alarm fatigue – the condition in which device users are repeatedly bothered by frequent and/or false alarms can be observed. Also, there can be skin irritation due to the constant presence of sensors on/in the body.
To counterbalance this restrain, more user-friendly devices should be prepared that will be easy to learn and comfortable to wear.
Lack of reimbursement policies
Price associated with continuous glucose monitoring devices is very high. These devices are not reimbursed by government schemes which may inhibit the growth of the market. For instance, according to an article published by the International Journal of Nursing Practice on April 13, 2023, Social Health Insurance system in China does not cover blood glucose testing tools. The glucose management course is also expensive and not reimbursable.
To counterbalance this restraint, more reimbursement policies should be introduced by the government.
China Continuous Glucose Monitoring Devices Market - Key Players
Major players operating in the China continuous glucose monitoring devices market include Medtronic PLC, Abbott Laboratories, Medtrum Technologies, Inc., Senseonics, Nemaura, STMicroelectronics, NXP Semiconductors, Qualcomm, Taiwan Semiconductor Manufacturing Company Limited, GE Healthcare, Microchip Technology Inc., Texas Instruments Inc., Micron Technology Inc., Renesas Electronics Corporation, and Toshiba Corporation.
*Definition: Continuous glucose monitoring (CGM) devices provide insights into the glucose levels of diabetic patients throughout the day. CGM devices are mostly used by patients with type 1 and type 2 diabetes for better glucose control to improve health and quality of life. Moreover, the integration of smartphones for easy diabetes management and cloud-based data storage are key features of the currently available CGM devices which have contributed to the drastic adoption of the CGM systems. The CGM devices display a complete picture of glucose trends with a graphical display and customizable alerts to indicate the disturbances in glucose status.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients