Childhood Absence Epilepsy Treatment Market - Insights
Absence seizures (previously known as petit-mal) are commonly found in children than in adults with frequent occurrence. The seizures of childhood absence epilepsy often starts between the ages of 4 to 7 years. It rarely starts before 4 years of age and late as 8 years of age. Seizures can occur many times a day ranging from 10 to 100 times a day, known as pyknolepsy. Seizures are divided into typical and atypical absence seizures depending on the nature of the seizures. As symptoms of some other medical conditions are similar to epilepsy, diagnosing epilepsy proves to be difficult. Some protocols should be followed by the patient such as maintaining a diary of seizure occurrence dates, time, and detailed description for easier diagnosis. Seizures should be diagnosed and classified by seizure type and syndrome. Ethosuximide is generally the first choice of treatment for childhood absence epilepsy Ethosuximide is very effective in typical absence seizures. Although Ethosuximide and Valproate are found to be similarly effective, Ethosuximide is better accepted by patients. Atypical absence seizures have been found to respond well to Valproic acid. Lamotrigine is considered after both the drugs Ethosuximide and Valproate, as lamotrigine is found to have low efficacy than the other drugs.
High prevalence of epilepsy and seizures among children is expected to drive global childhood absence epilepsy treatment market growth over the forecast period
Epilepsy is a highly prevalent disorder, especially in emerging economies. The incidence and prevalence of epilepsy in children appears to be lower in developed economies and highest in rural areas of underdeveloped economies. For instance, according to the World Health Organization (WHO), February 2018, around 80% of the people suffering from epilepsy live in low– and middle- income countries. According to the American Academy of Pediatrics in April 2017, epilepsy affects 0.5% to 1% of children, globally and is the most frequent chronic neurologic condition observed in childhood. Only around one-third of children with epilepsy can be assigned to a specific epilepsy type, as defined by the recently proposed system for classification of epilepsy syndrome (ILAE Classification of epilepsies). According to the Society of Neuroscience in 2015, epilepsy affects an estimated 10.5 million children worldwide, and the types and severity of the disorder vary significantly. According to the National Center for Biotechnology Information (NCBI), 2013, around 10% of seizures in children with epilepsy are typical absence seizures.
The global childhood absence epilepsy treatment market was valued at US$ 158.2 million in 2017 and is expected to witness a robust CAGR of 5.4% over the forecast period (2018 – 2026).
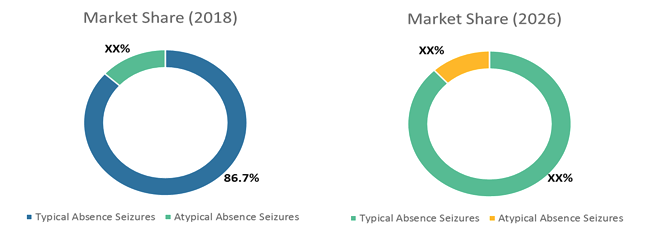
Figure 1: Global Childhood Absence Epilepsy Treatment Market Share, By Disease Type (%), 2018 - 2026
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2017)
Increasing focus of market players to target the unmet needs of absence epilepsy patients is expected to boost childhood absence epilepsy treatment market growth over the forecast period
Childhood absence epilepsy starts between the ages of 4 to 7 years, rarely the seizures may start under 4 years of age and late as 8 years of age. Manufacturers are engaged in research and development of new drugs for the treatment of childhood absence epilepsy. Furthermore, pipeline of therapeutics for other forms of pediatric epilepsy is strong and epilepsy medications are majorly effective for the treatment of more than one type of seizure. Presence of strong pipeline for overall pediatric epilepsy may result in growth in global childhood absence epilepsy treatment market size. Some of the market players involved in the research and development of drugs for childhood absence epilepsy include INSYS Therapeutics Inc. and Cavion, Inc. The market entry strategy for pipeline drugs is based on initially seeking approval for multiple indications including childhood absence epilepsy. Insys Therapeutics has Cannabidiol oral solution for the treatment of childhood absence epilepsy and various other indications in phase 2 of clinical trials (estimated study completion date is November 2019). Cavion, Inc. has CX-8998 for the treatment of childhood absence epilepsy in phase 2 clinical trials (estimated study completion date is November 25, 2018).
However, side effects associated with these medications are expected to restrain global childhood absence epilepsy treatment market growth to a certain extent. The anti-epileptic drugs show mild neurological adverse effects and at times, it can be fatal. The adverse reactions and risks vary with each medication. Majority of the anti-epileptic drugs have the potential to cause an allergic reaction.
Some of the major players operating in the global childhood absence epilepsy treatment market include Cavion, Inc., Pfizer, Inc., GlaxoSmithKline Plc, Insys Therapeutics, Inc., AbbVie, Inc., and Teva Pharmaceutical Industries Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients