Cervical Total Disc Replacement Device Market is estimated to be valued at USD 3.56 Bn in 2025 and is expected to reach USD 14.16 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 21.8% from 2025 to 2032. Degenerative disc disease (DDD) is a common cause of neck discomfort. The main danger of acquiring DDD is becoming older. The prevalence of cervical degenerative disc disease (DDD) has grown in tandem with the aging of the global population. The incidence of cervical spine procedures in the U.S. has significantly grown, despite the fact that the initial therapy is often conservative. In the past, anterior cervical discectomy and fusion (ACDF) was thought to be the only surgical option for cervical spine DDD symptoms. The objectives of ACDF are to decompress the spinal nerves to release pressure on them and to realign and stabilize the spinal column. A novel surgical technique called cervical disc replacement (CDR) has recently come to light as a potential substitute for anterior cervical discectomy and fusion (ACDF), the more popular decompressive treatment in the cervical spine.
Analysts’ Views on Global Cervical Total Disc Replacement Device Market:
The global cervical total disc replacement device market is expected to grow significantly by 2032 due to increasing organic growth strategies adopted by the key players such as commercializing the use of cervical total disc replacement system. For instance, in September 2022, Centinel Spine, Inc., one of the leading global medical device companies addressing cervical and lumbar spinal disease, announced the first implantation of its prodisc C Vivo Cervical Total Disc Replacement (TDR) product in the Western U.S. Prodisc C Vivo Cervical Total Disc Replacement (TDR) is a device intended to help restore the natural distance between two vertebrae and the natural motion of the lumbar spine. Thus, the increasing adoption of organic growth strategies by key players will help expand the global cervical total disc replacement device market growth by 2032.
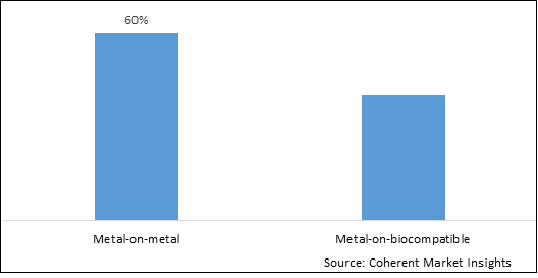
Figure 1. Global Cervical Total Disc Replacement Device Market Share (%), by Material, 2025
To learn more about this report, Download Free Sample
Global Cervical Total Disc Replacement Device Market- Drivers
Increasing strategies by the research institutes for increasing the pool of neurosurgeons in U.S. to drive the market growth during the forecast period
Increasing strategies by the research institutes for increasing the pool of neurosurgeons in U.S. is expected to drive the global cervical total disc replacement device market growth by 2032. For instance, on January 24, 2023, the American Society for Stereotactic and Functional Neurosurgery (ASSFN), a research institute focused on using the full potential of functional neurosurgery to improve patients’ lives through education, collaboration, innovation, and advocacy, announced that the Cerebrovascular Section of the American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) and the American Society for Stereotactic and Functional Neurosurgery (ASSFN) engaged in promoting the just-launched Neurosurgeon-Scientist Training Program (NSTP) of the Society of Neurological Surgeons (SNS). The NSTP was created by the SNS to expand the number of neurosurgery residents engaged in research and raise their likelihood of becoming self-sufficient neurosurgeon-scientists. For neurosurgery residents who are starting their protected research year or have recently finished their protected research year, the NSTP will act as a structured supervised research program.
Increasing product approvals by the U.S. Food and Drug Administration
Increasing organic strategies such as product approvals by the U.S. FDA is expected to propel the market growth during the forecast period. For instance, in July 2022, Centinel Spine, Inc. announced the U.S. Food and Drug Administration (FDA) Pre-Market Application (PMA) approval of 1-level indications for three additional cervical total disc replacement (TDR) devices: prodisc C Vivo, prodisc C Nova, and prodisc C SK. Prodisc C Vivo has keel-less endplates including a convex, superior endplate to match more concave vertebral anatomy, while prodisc C SK and prodisc C Nova implant designs have flat endplates with low-profile keels to better match flat vertebral anatomy.
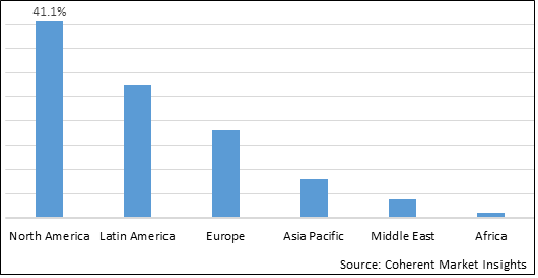
Figure 2. Global Cervical Total Disc Replacement Device Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Cervical Total Disc Replacement Device Market- Regional Analysis
Among regions, North America is expected to hold a dominant position in the global cervical total disc replacement device market over the forecast period. North America is estimated to hold 41.1% market share in 2025 due to the increasing adoption of organic growth strategies such as pre-market approval, which is expected to propel the market growth during the forecast period. For instance, in March 2019, MCRA, LLC, one of the leading adviser and clinical research organizations to the neuro-musculoskeletal and orthopedic industry, announced its role in the successful Premarket Approval (PMA) application decision by the U.S. Food and Drug Administration (FDA) to approve Orthofix M6-C artificial cervical disc for the treatment of single-level cervical disc degeneration. M6-C disc, a next-generation artificial cervical disc created by Spinal Kinetics LLC, is the only artificial cervical disc intended to closely resemble a natural disc's anatomical structure. MCRA was first hired by Spinal Kinetics, a medical company using Board Certified Radiologists use CRMA (radiographic mensuration criteria in 2008 during the preliminary phases of creating the clinical research approach for M6-C artificial cervical disc, which was later bought by Orthofix Medical Inc. one of the leading medical device company and provider of spinal, orthopedic, bone growth, and motion preservation products in 2018. It took 15 months for the FDA to analyze and approve the PMA for the M6-C disc after it was submitted on February 6, 2019. Furthermore, M6-C artificial cervical disc's PMA certification is the first original PMA approval for a spinal device since 2015.
Moreover, research and development of cervical total disc replacement technology is expected to offer lucrative growth opportunities to market players in Europe. For instance, in September 2019, researchers from the Institute for Biomechanics, Switzerland reported the development of a method for controlled epigallocatechin 3-gallate delivery in the degeneration of the intervertebral disc. The approach is expected to restore the homeostasis of the degeneration of the intervertebral disc. Moreover, the availability of CE marked products such as Freedom Cervical Disc developed by AxioMed LLC, a company focused on developing first-generation artificial discs in the region is contributing to the Europe cervical total disc replacement device market growth.
Cervical Total Disc Replacement Device Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.56 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 21.8% | 2032 Value Projection: | USD 14.16 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Stryker Corporation, Medtronic plc., Zimmer Biomet Holdings, Inc., Globus Medical, Inc., FH Orthopedics, Orthofix Medical Inc., NuVasive Inc., Centinel Spine, Inc., ZimVie, inc., and other prominent players.and other prominent players. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Cervical Total Disc Replacement Device Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020. According to the World Health Organization’s report, the manifestation of coronavirus (COVID-19) has resulted in more than 767,984,989 confirmed cases infected by COVID-19 worldwide as of June 2023.
COVID-19 has affected the economy in three main ways: by directly affecting the production and demand of drugs and vaccines, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, U.A.E., Egypt, and others faced problems with the transportation of drugs and vaccines from one place to another.
Moreover, there was a significant impact of the COVID-19 pandemic on the utilization of common spine procedures and percentages of outpatient procedures. For instance, data was published by the National Center for Biotechnology Information on May 2, 2023, according to which the COVID-19 pandemic had the most effect on the amount of spine surgeries in March and April 2020. Despite the fact that total procedures fell by 4.3% from 2019 to 2020.
Global Cervical Total Disc Replacement Device Market Segmentation:
The global cervical total disc replacement device market report is segmented into material, design, end user, and region.
Based on Material, the market is segmented into metal-on-metal and metal-on-biocompatible. Out of which, the metal-on-biocompatible segment is expected to hold a dominant position in the global cervical total disc replacement device market during the forecast period, and this is attributed due to improvement for the patient compared to conventional fusion surgery.
Based on Design, the market is segmented into constrained, semi-constrained, and unconstrained. Out of which, the semi-constrained segment is expected to dominate the market over the forecast period and this is attributed due to its extensive researched type of prosthesis.
Based on End User, the market is segmented into Hospitals, Ambulatory Surgical Center and Specialty Clinics, and Clinics. Out of which, the Hospitals segment is expected to dominate the market over the forecast period and this is attributed to increasing cervical disc replacement surgeries in hospitals.
Based on Region, the market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, North America is expected to have a dominant position in the global cervical total disc replacement device market during the forecast period and this is attributed to increasing adoption of organic growth strategies such as pre-market approval.
Global Cervical Total Disc Replacement Device Market Cross Sectional Analysis:
In design segment, the semi-constrained segment held a dominant position in North America due to its extensive researched type of prosthesis. For instance, in September 2021, according to data provided by the Journal of Orthopaedic Surgery, semi-constrained prosthesis was most widely used during surgery because it was the most extensively researched type of prosthesis. Although there is little distinction between prosthesis types, it is advised that during cervical arthroplasty with keel devices, a generous exposure be used which is done using semi-constrained prosthesis.
Global Cervical Total Disc Replacement Device Market: Key Developments
On February 14, 2023, Centinel Spine, LLC, a privately-held spine company focused on anterior column reconstruction, announced the completion of the 500th procedure in the U.S. with its latest US FDA-approved total disc replacement (TDR) system of prodisc cervical solutions, prodisc C Vivo, and prodisc C SK. The keel-less prodisc C Vivo device offers a combination of a well-designed anatomical endplate shape along with robust spikes that provide strong immediate fixation.
On January 30, 2023, ZimVie Inc., one of the global life sciences leaders in the dental and spine markets, announced that over 200,000 Mobi-C Cervical Discs have been implanted worldwide. Since the device's first operation was done in France in 2004, this comprises patients who have been treated in more than 25 nations. The U.S. Food and Drug Administration granted Mobi-C clearance as the first cervical disc in 2013 to treat several levels of the cervical spine.
In November 2019, Orthofix Medical Inc., one of the leading medical device company and provider of spinal, orthopedic, bone growth, and motion preservation products received the U.S. Food and Drug Administration approval for its M6-C artificial cervical disc for patients with cervical disc degeneration. The device was originally developed by Spinal Kinetics; however, Spinal Kinetics was acquired by Orthofix in March 2018.
In March 2019, AxioMed LLC, a medical device company specialized in the development of spine technology for fusion and first-generation artificial discs, received the Conformité Européenne (CE) mark for its Cervical Freedom Total Disc Replacement, a viscoelastic disc replacement device.
Global Cervical Total Disc Replacement Device Market: Key Trends
Increasing research and development activities
Increasing research and development activities is expected to drive the market growth over the forecast period. For instance, on January 6, 2023, The Disc Replacement Center along with Mr. Armen Khachatryan (MD), Doctor of Medicine and a skilled provider specializing in Orthopedic Surgery near West Jordan UTannounced the successful completion of the first series of MOTUS lumbar total joint replacement surgeries as part of the BalancedBack pivotal clinical trial sponsored by 3Spine, Inc., a private, clinical phase healthcare company. In a second prospective 3Spine research, patients who underwent a standard spinal fusion were compared to patients who underwent the innovative low back complete joint surgery to see how they fared. At the Global Microsurgical Center of Park City, Dr. Khachatryan finished the initial set of MOTUS operations. In the following months, he will accept a small number of additional patients from the wider Salt Lake City region.
Global Cervical Total Disc Replacement Device Market: Restraint
High rates of complications and rehospitalizations after cervical disc replacement surgery
High rates of complications and rehospitalizations after cervical disc replacement surgery can hinder the market growth over the forecast period. For instance, in March 2020, National Center for Biotechnology Information published a data according to which there are high rate of certain complications after the cervical disc replacement surgeries (CDR). In the first postoperative year following CDR, mechanical and/or bone-related complications were the most prevalent (reported in 12.3% of patients), and they included implant mispositioning, heterotrophic ossification, and neighboring disc degeneration. Moreover, 4% of CDR patients required further surgery as a result of device misalignment, migration, subsidence, or persistent discomfort. At 4 years after surgery, a second meta-analysis reported a 7.4% incidence of neck discomfort, implant migration, or radiculopathy which is going to impede the growth of cervical disc replacement device market during the forecast period. But the high rates of complications in hospitals and rehospitalization after the surgery can be avoided by proper care after the surgery.
Global Cervical Total Disc Replacement Device Market- Key Players
Major players operating in the global cervical total disc replacement device market include Stryker Corporation, Medtronic plc., Zimmer Biomet Holdings, Inc., Globus Medical, Inc., FH Orthopedics, Orthofix Medical Inc., NuVasive Inc., Centinel Spine, Inc., ZimVie, inc., and other prominent players.
Definition: Cervical total disc replacement (TDR) is a procedure done to alleviate neck discomfort and neurological problems brought on by cervical spine disc degeneration.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients