Cervical Cancer Diagnostic Tests Market is estimated to be valued at USD 7.95 Bn in 2025 and is expected to reach USD 12.19 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.3% from 2025 to 2032.
Analysts’ Views on Global Cervical Cancer Diagnostic Tests Market:
Over the projected period, increased teenage sexual activity, rising incidence of cervical cancer, and high prevalence of (human papillomavirus) HPV-infected individuals are anticipated to drive the growth of the global market for cervical cancer diagnostic tests. For instance, in February 02, 2023, according to the American Society of Clinical Oncology (ASCO), globally an estimated 604,127 women were diagnosed with cervical cancer in 2020 and about 341,831 women worldwide died from cervical cancer.
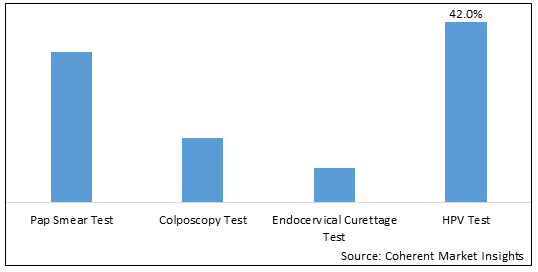
Figure 1. Global Cervical Cancer Diagnostic Tests Market Share (%), By Test Type, 2025
To learn more about this report, Download Free Sample
Global Cervical Cancer Diagnostic Tests Market– Drivers
High prevalence of human papillomavirus (HPV) infected patients
Increasing prevalence of HPV infected patients is expected to the growth of global cervical cancer diagnostic tests market over the forecast period. For instance in June 2021, according to the National Cancer Institute, each year, according to the Centers for Disease Control (CDC) estimates that HPV causes around 36,000 of the approximately 45,000 new cases of cancer in body regions where it is frequently prevalent. High-risk HPVs cause about 5% of all cancers worldwide, with an estimated 570,000 women and 60,000 men getting an HPV-related cancer each year.
Increasing awareness about the cervical cancer diagnosis
Increasing awareness about the cancer diagnosis is expected to drive the market over the forecast period. For instance, in January 23 2023, in 2020, the World Health Organization (WHO), set a goal to eliminate cervical cancer as a public health problem globally by 2120. To reach this goal, WHO Member States of North America, Europe, Latin America, Asia Pacific, Middle East and Africa should strive to meet the following interim scale-up targets by 2030: 90% of girls are fully vaccinated with human papillomavirus (HPV) vaccine by 15 years of age; 70% of women are screened using a high-performance test by 35 years of age and again by 45 years of age; 90% of women with pre-cancer are treated, and 90% of women with invasive cancer are managed. To build on the momentum of the global strategy to accelerate the elimination of cervical cancer as a public health problem, a regional cervical cancer elimination strategy has been developed for the WHO Eastern Mediterranean Region that is adapted to the religious, cultural, social, economic and geographical contexts in the Region.
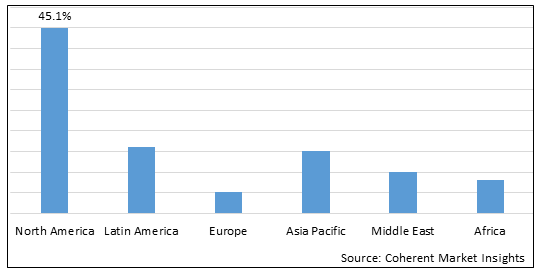
Figure 2. Global Cervical Cancer Diagnostic Tests Market Value (US$ Billion), By Region, 2025
To learn more about this report, Download Free Sample
Global Cervical Cancer Diagnostic Tests Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global cervical cancer diagnostic tests market over the forecast period owing to the increase in cervical cancer prevalence. For instance, on June 08, 2023, according to the Centers for Disease Control and Prevention, each year in the U.S, about 11,500 new cases of cervical cancer are diagnosed and about 4,000 women die of this cancer.
Global Cervical Cancer Diagnostic Tests Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global cervical cancer diagnostic tests market owing to the decrease and delay in cancer screening and also by directly affecting the production and demand, by creating disruptions in distribution channels, and through its financial impact on companies and financial markets. According to the study published in the Diagnostics Journal in April 2022, during the first lockdown in April 2020, the number of tests dropped by 75.5%, after which the number of cases dropped by up to 36.1% in 2021. During the first 24 months of the pandemic, the total volume of tests lost was 49.9%. The percentage of late-stage cervical cancers (stages III–IV) increased by 17%, while the number of newly diagnosed cancers in the outpatient clinic decreased by 45% from the baseline. However, the market growth is stabilizing in the current scenario after COVID-19 as the worldwide restrictions have eased and disease screening services have been resumed.
Cervical Cancer Diagnostic Tests Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.95 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.3% | 2032 Value Projection: | USD 12.19 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Guided Therapeutics, Inc., Abbott, Beckman Coulter, Inc., BD (Becton, Dickinson and Company), F. Hoffmann-La Roche Ltd, Bio Farma, Hologic, Inc., Oncgnostics GmbH, AstraZeneca, MobileODT, Agilent Technologies, Inc., Thermo Fisher Scientific Inc., DYSIS Medical Inc., The Cooper Companies Inc., Danaher, Quest Diagnostics, Arbor Vita Corporation, and Zilico. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Cervical Cancer Diagnostic Tests Market- Segmentation
The global cervical cancer diagnostic tests market report is segmented into test type, age group, and region.
Among Test Type, the global cervical cancer diagnostic tests market is segmented into Pap smear test, colposcopy test, endocervical curettage test, and HPV test. Out of which, the HPV Test segment is expected to dominate the global cervical cancer diagnostic tests market during the forecast period and this is due to the presence of Human Papillomavirus (HPV) infection and availability of homes based sample collection kits of HPV test.
Among Age Group, the global cervical cancer diagnostic tests market is segmented into age 20–40 and age above 40. The age above 40 segment is expected to dominate the global cervical cancer diagnostic tests market over the forecast period owing due the number of people who have had several partners has increased and long-lasting infection with certain types of human papillomavirus (HPV).
Among Region, the global cervical cancer diagnostic tests market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, the North America is expected to dominate the market over the forecast period owing to the increasing prevalence of cervical cancer and high prevalence of human papillomavirus (HPV) infected patients.
Among all segmentation, the test type segment has the highest potential due to the increasing HPV test due to the rise in prevalence of HPV infected patients over the forecast period. For instance, in June 2022, Karkinos Healthcare, an oncology-focused health-tech platform, launched CerviRaksha, a first-of-its-kind clinically validated HPV test that is prequalified by the World Health Organization and approved by the U.S Food and Drug Administration in partnership with the Karkinos network hospital doctors and nurses.
Global Cervical Cancer Diagnostic Tests Market- Cross Sectional Analysis
Among Test type, the HPV test segment held a dominant position in North America region over the forecast period due to increasing number of HPV infected patients. For instance, in June 2022, according to the Centers for Disease Control and Prevention (CDC), from 2016 to 2020, about 47,199 new HPV-associated cancers occur in the U.S each year: 26,177 among women, and 21,022 among men. HPV-associated cancers are estimated by examining cancer in parts of the body and cancer cell types that are more likely to be caused by HPV.
Global Cervical Cancer Diagnostic Tests Market- Key Developments
On March 07 2023, Roche Products (India) Pvt. Ltd., a suppliers & service providers of Diagnostic & Therapeutic products, announced the signing of a Memorandum of Understanding (MoU) with the Cancer Awareness Prevention and Early Detection Trust (CAPED), a non-profit organisation dedicated to promoting cancer awareness, prevention, and early detection. The partnership with CAPED is part of Roche’s broader commitment to improving cancer care globally. The company has a long-standing legacy of innovation in cancer diagnostics and therapies, and has been at the forefront to develop new approaches to cancer care.
In April 2021, Hologic, Inc., an innovative medical technology company announced that its new Genius Digital Diagnostics System is now commercially available in Europe. The Genius Digital Diagnostics System is the next generation of cervical cancer screening that combines deep learning-based artificial intelligence (AI) with advanced volumetric imaging technology to help identify pre-cancerous lesions and cervical cancer cells in women. It was developed to provide actionable insights, and improve workflow and lab efficiency, all with one goal in mind – to eradicate cervical cancer.
In November 2022, the (Malinauskas) Australia Government, has launched a ‘You Can Do It’ campaign to encourage women aged 25 to 74 to consider self-collection for its five yearly screening. Self-collection is all about giving women choice and control. Wellbeing SA (Sothern Australia), a state government agency leading a focus and action on prevention has also partnered with Cancer Council to fund training for (General Practitioner) GPs in self-collection to ensure women across the state are supported to choose the best option for them when it comes to cervical screening.
In May 2022, U.S. Department of Health and Human Services (HHS), announced the availability of US$ 5 million for community health centers, funded by HHS’s Health Resources and Services Administration, to increase equitable access to life-saving cancer screenings. This funding supports President Joe Biden’s Unity Agenda and his call to action on cancer screening and early detection as part of the Administration’s Cancer Moonshot initiative to end cancer as we know it.
Global Cervical Cancer Diagnostic Tests Market- Key Trends
Colposcopy is expected to grow over the forecast period
A colposcopy is a test to examine the cervix more closely. The cervix is the vaginal opening into the womb. If a cervical screening reveals cell alterations brought on by specific forms of human papillomavirus (HPV), a colposcopy is frequently performed. The Colposcopy test provide high accuracy as compared to the Pap smear test. For instance, in October 2020, precision of the Pap smear was 72.2%. Sensitivity and specificity of colposcopy were 66.7% (CI: 60.7– 72.7) and 98.94% (CI: 92.94–100), respectively, and the positive and negative predictive values of colposcopy were 80 and 97.9%, respectively. In general, the accuracy of colposcopy was calculated as 97%.
Global Cervical Cancer Diagnostic Tests Market: Restraints
Lack of spousal and family support
Lack of spousal and or family support is expected to hinder the market growth over the forecast period For instance, in December 2022, according to an article published in the Journal of Biomed Central, lack of spousal and or family support were key barriers, and these may be driven by misconceptions about cervical cancer and traditional, cultural, or religious beliefs about pelvic examination and cancers, and this has also been reported in high income countries such as U.S, Australia, Germany, etc. Overlapping with cultural/traditional and religious barriers were other social factors including misconceptions and stigmatization of screening and cervical cancer, largely shaped by gender norms. So, rise in the awareness campaigns cab ne imitated to avoid this unawareness.
Global Cervical Cancer Diagnostic Tests Market- Key Players
Major players operating in the global cervical cancer diagnostic tests market include Guided Therapeutics, Inc., Abbott, Beckman Coulter, Inc., BD (Becton, Dickinson and Company), F. Hoffmann-La Roche Ltd, Bio Farma, Hologic, Inc., Oncgnostics GmbH, AstraZeneca, MobileODT, Agilent Technologies, Inc., Thermo Fisher Scientific Inc., DYSIS Medical Inc., The Cooper Companies Inc., Danaher, Quest Diagnostics, Arbor Vita Corporation, and Zilico.
*Definition: The human papilloma virus (HPV) is tested for during a cervical screening (smear test) on a sample of cervix cells. If someone has a particular high-risk type of HPV, they should be examined for any cellular alterations. Cervical cancer might later arise from these modifications. A smear test for cervical screening evaluates the condition of the cervix. The cervix is the vaginal opening to the womb.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients