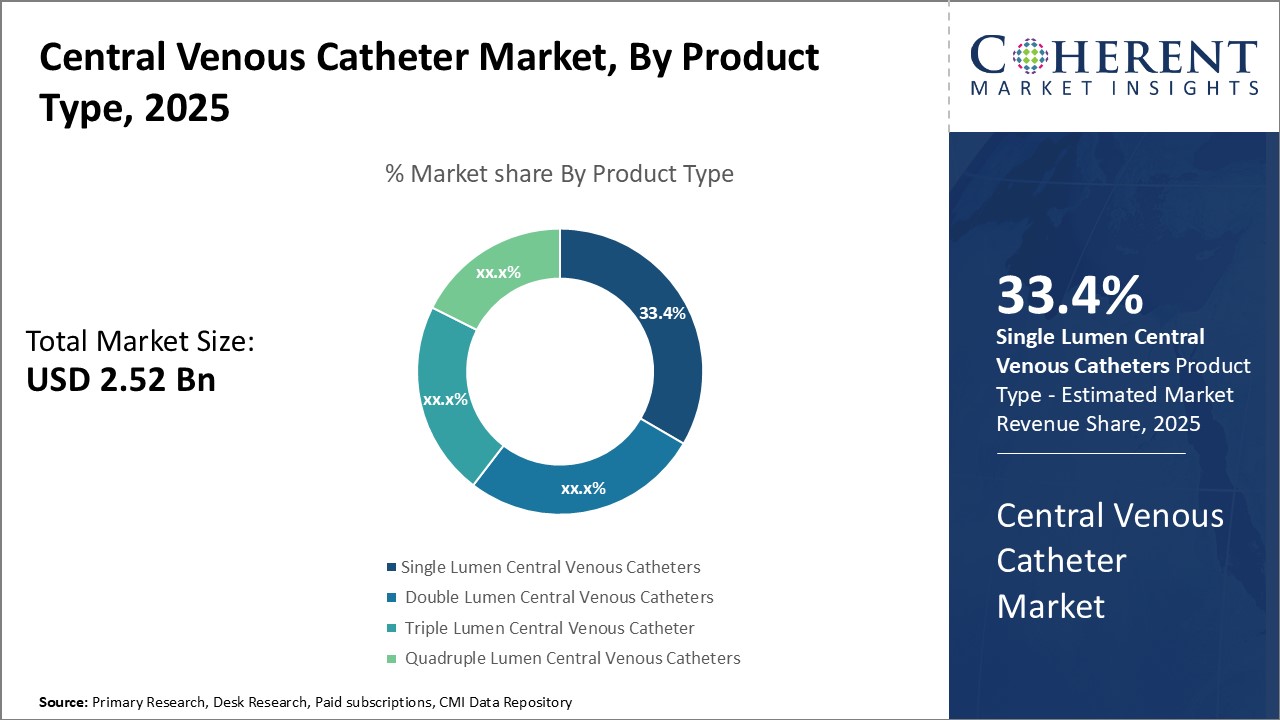
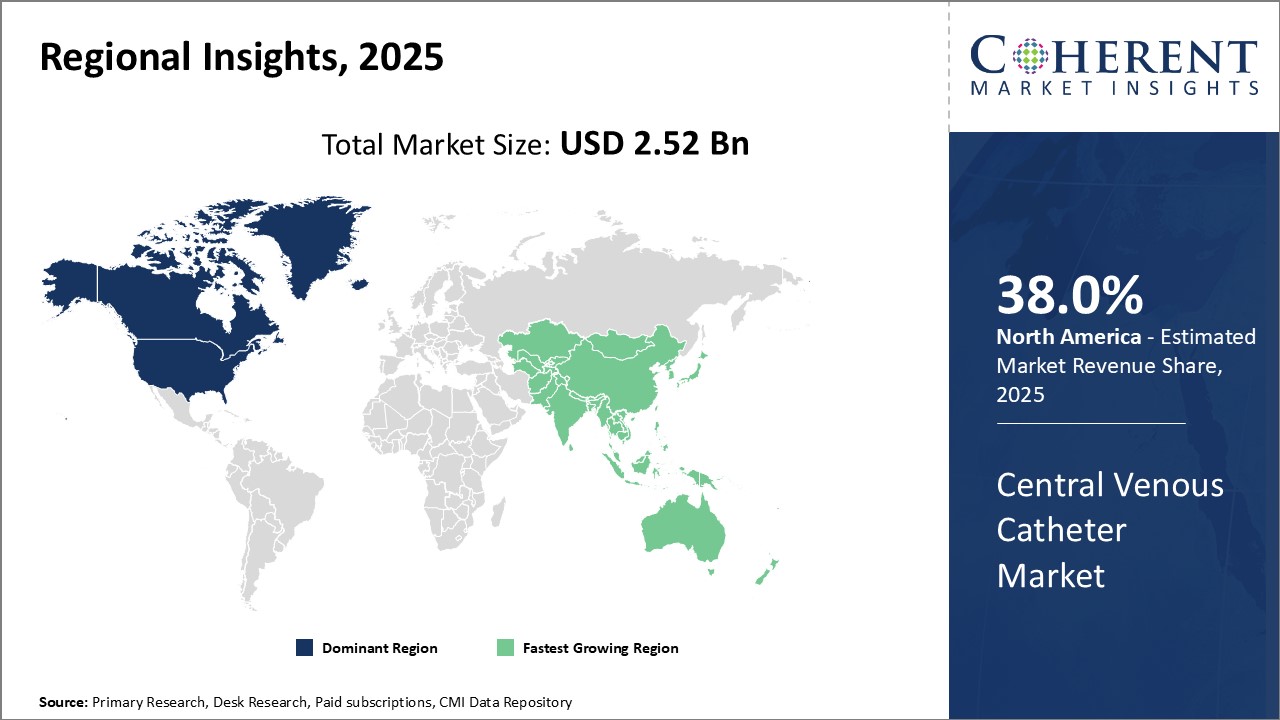
The global central venous catheter market is estimated to be valued at USD 2.52 Bn in 2025 and is expected to reach USD 4.03 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.9% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
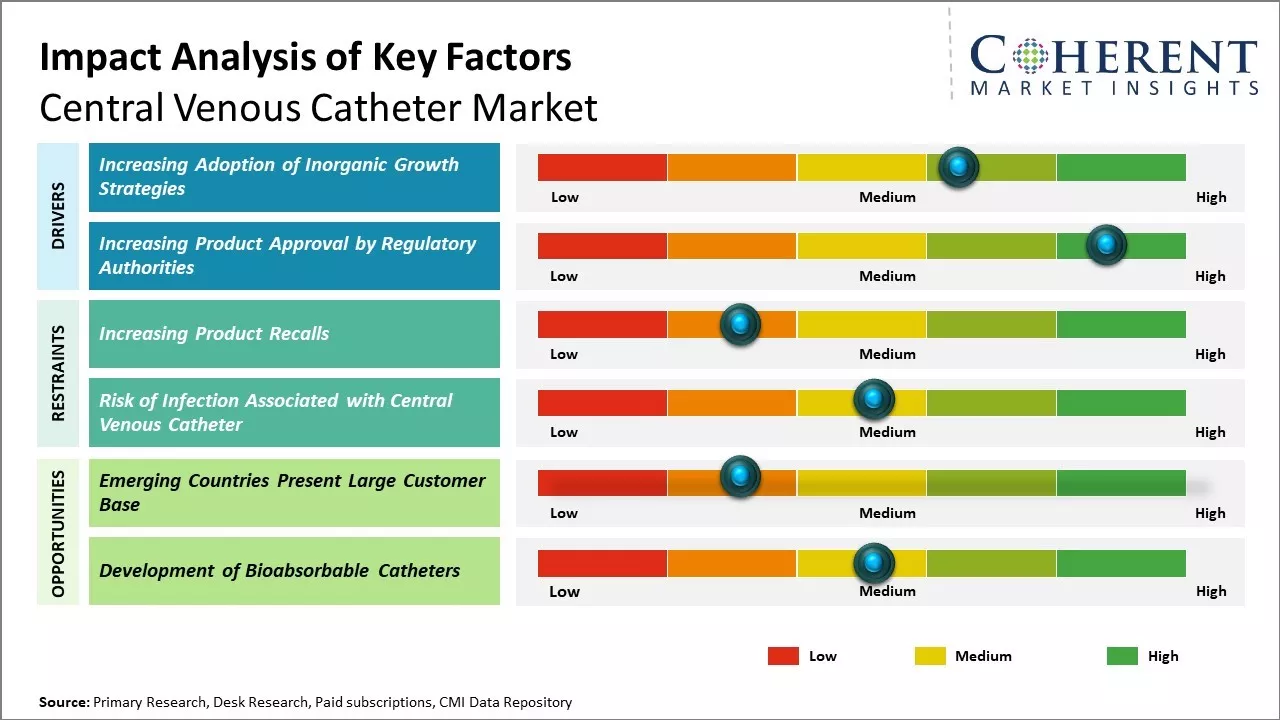
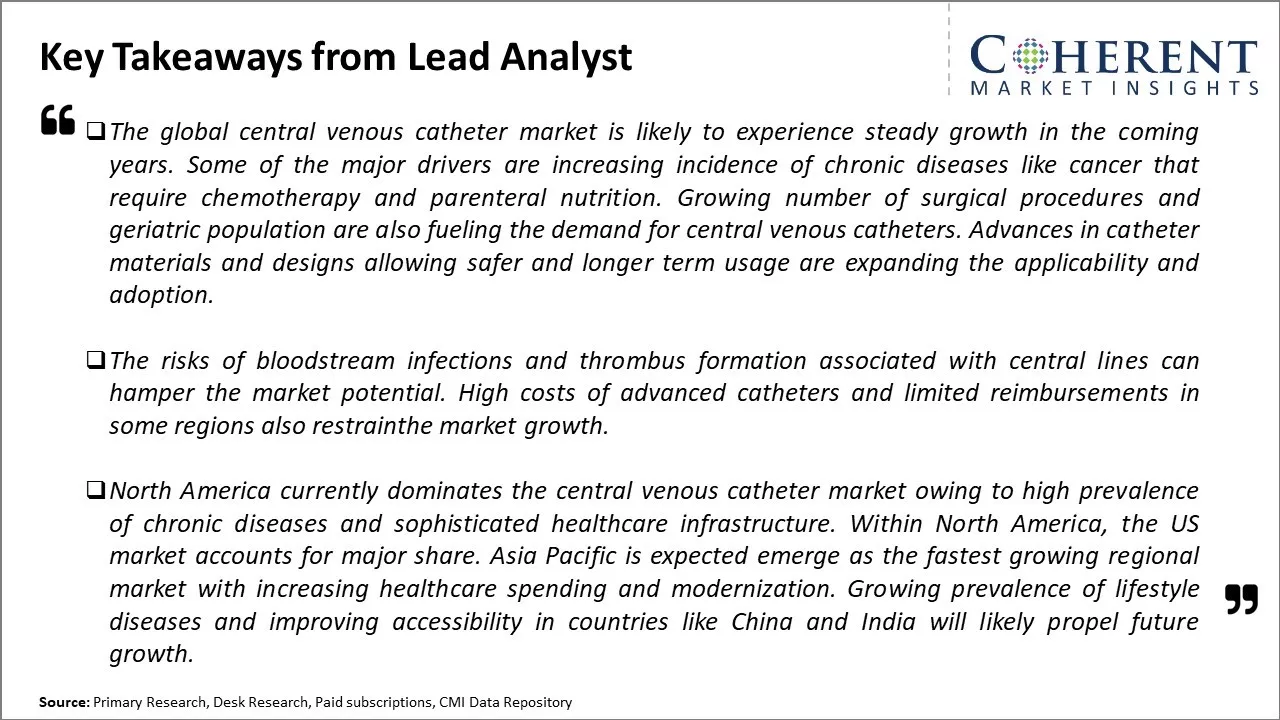
The global central venous catheter market is driven by the rising prevalence of chronic and lifestyle diseases. Long-term usage of catheters in homecare settings and ambulatory surgical centers is also expected to support market growth. However, risks of bloodstream infections, deep vein thrombosis associated with central line placement, and poor reimbursement policies in developing nations may hamper the market growth during the forecast period.
Market Driver – Increasing Adoption of Inorganic Growth Strategies
The increasing adoption of inorganic activities by market players such as signing distribution agreements is expected to drive the market growth over the forecast period. For instance, in August 2021, ZOLL Medical, a medical device company, and Wallaby Medical Technologies, a medical device company dedicated to stroke treatment, announced a distribution agreement, in which Wallaby will manage the sales and distribution of ZOLL Medical's temperature management products in China. The Thermogard XP Intravascular Temperature Management System (IVTMTM) provides healthcare providers with the precision and speed associated with high-quality targeted temperature management (TTM), resulting in clinically demonstrated improvements in neurological outcomes among cardiac arrest survivors. The system includes a family of heat-exchange catheters that allow clinicians to manage core body temperature based on the patient’s needs.
Get actionable strategies to beat competition: Download Free Sample
Increasing Product Approvals by Regulatory AuthoritiesIncreasing adoption of growth strategies, such as product approvals by regulatory authorities such as the U.S. Food and Drug Administration, is expected to drive the market growth over the forecast period. For instance, in March 2020, Access Vascular, Inc., a medical device manufacturer, received the U.S. Food and Drug Administration (FDA) clearance for the second generation of its HydroPICCTM peripherally inserted central catheter (PICC).
To learn more about this report, Download Free Sample
Market Challenge– Increasing Product RecallsThe increasing product recalls is expected to hamper the growth of the global central venous catheter market over the forecast period. For instance, in January 2020, ICU Medical, Inc., a company manufacturing medical technologies, initiated Class 2 device recall of heparin coated central venous catheter due the inability of the guidewire to pass through the needles included with the catheter kits. Moreover, in January 2019, Arrow International Inc., a part of the Teleflex Inc., a medical device company, initiated the Class 2 Device Recall of Arrow QuadLumen central venous catheterization kits, as the product contained incorrect banner card within the kit.
Market Opportunity – Development of Bioabsorbable Catheters
The development of bioabsorbable catheters represents a major opportunity in the global central venous catheter market. Bioabsorbable catheters are made from materials that can dissolve and disappear in the body over time without needing subsequent removal procedures. This offers several advantages over traditional plastic catheters. Currently, most central venous catheters are made of non-absorbable plastics like polyurethane and silicon. While effective, these catheters must be surgically removed once therapy is completed. This subjects patients to additional risks of complications from invasive procedures. It also increases the length of hospital stay and healthcare costs. In contrast, bioabsorbable catheters eliminate the need for catheter removal procedures as the material is absorbed safely by the body. This makes them particularly beneficial for short-term applications where the risk of complications from removal may outweigh the benefits.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Product Type: Convenience and cost-effectiveness drive the demand for single-lumen cathetersThe product type segment includes, single lumen central venous catheters, double lumen central venous catheters, triple lumen central venous catheter, and quadruple lumen central venous catheters. The single lumen central venous catheters sub-segment is estimated to hold 33.4% of the market share in 2025 owing to its convenience and lower cost compared to multi-lumen catheters. Single-lumen catheters are ideal for simple procedures that require administration of one fluid or medication at a time. They are easy to insert with a single puncture point, minimizing the risk of complications. Their simple design also makes them more affordable for healthcare providers and patients compared to double, triple, or quadruple lumen catheters. This has boosted their uptake significantly, especially in developing markets where budgets are more constrained. Moreover, for short-term uses where multiple infusions are not required concurrently, single-lumen catheters suffice and their use avoids additional costs. Their convenience and affordability continue to drive their leading market dominance over other product types.
Insights, By Application: Specialized applications fuel the demand for multi-lumen catheters
The application segment includes chemotherapy, drugs and fluid administration, blood transfusions, and others. The chemotherapy sub-segment is estimated to hold 52.8% of the market share in 2025. This can be attributed to the complex treatment requirements of chemotherapy that necessitate the use of multi-lumen catheters. Chemotherapy often requires simultaneous administration of multiple drugs, fluids, and blood sampling over extended periods. This is best facilitated through multi-lumen catheters like double or triple lumen catheters that accommodate multiple infusions concurrently through separate lumens while minimizing the risks of extra punctures. Their specialized design makes them particularly suitable for complex chemotherapy protocols. As cancer incidence rises globally, chemotherapy applications are growing exponentially, driving the need for technologically advanced multi-lumen catheters that can optimally support intensive chemotherapy regimens.
Insights, By End User: Hospitals lead utilization driven by specialized patient care needs
The end user segment includes hospitals & clinics, ambulatory surgical centers, and others. The hospitals & clinics sub-segment is estimated to hold 43.2% of the market share in 2025. This is because hospitals treat a majority of critical patients requiring central venous access for complex therapies, intensive care, and long-term medication administration. They have advanced infrastructural and professional resources to handle high-risk procedures like central line insertions performed by skilled clinicians and specialists. Moreover, patients undergoing major surgeries or facing chronic illnesses tend to rely more on inpatient hospital care rather than outdoor facilities. As patients grow reliant on specialized therapeutics mainly available in hospitals, their catheter utilization rate is highest in these settings. Strong healthcare infrastructure and expertise in vascular access within hospitals have made them the largest end users of central venous catheters.
Need a Different Region or Segment? Download Free Sample
North America remains the dominant region in the global tissue engineering market and is estimated to hold 38.0% of the market share in 2025. This dominance can be attributed to the well-established healthcare infrastructure and high healthcare spending in countries like the U.S. The region is home to some of the largest medical device companies in the world with strong focus on innovation. Additionally, the reimbursement environment in North America is favorable for catheter procedures compared to other regions. This ensures steady demand for central venous catheters from hospitals and clinics.
The Asia Pacific region has emerged as the fastest growing market in recent years. The Asia Pacific central venous catheter market is driven by factors such as the rising geriatric population, improving access to healthcare facilities, and growing medical tourism industry. Countries like China, India, Japan, and South Korea are expected to be at the forefront of this growth. With economic development and rising incomes, the demand for advanced healthcare services is increasing rapidly. While availability and affordability still remain concerns for some segments, the overall scenario is quite promising for catheter device manufacturers seeking to tap into new opportunities.
Central Venous Catheter Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.52 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.9% | 2032 Value Projection: | USD 4.03 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
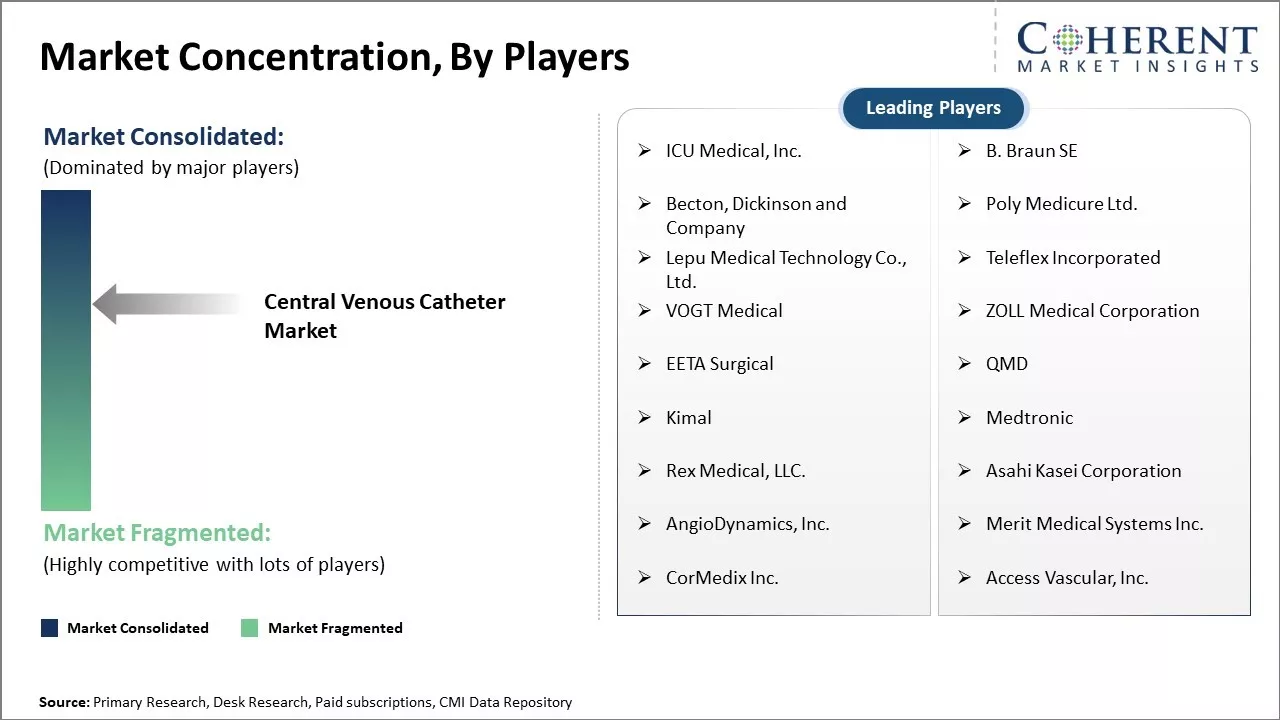
| Companies covered: |
ICU Medical, Inc., B. Braun SE, Becton, Dickinson and Company, Poly Medicure Ltd., Lepu Medical Technology Co., Ltd., Teleflex Incorporated, VOGT Medical, ZOLL Medical Corporation, EETA Surgical, QMD, Kimal, Medtronic, Rex Medical, LLC., Asahi Kasei Corporation, AngioDynamics, Inc., Merit Medical Systems Inc., CorMedix Inc., and Access Vascular, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: A central venous catheter is also known as central line or central venous line. It is a flexible tube, which is inserted into the vein below the right side of the collarbone and guided towards the large vein towards the heart, known as superior vena cava. A central venous catheter is used for administration of various fluids and these fluids can be easily injected into the body of the patients. The fluids include intravenous fluid, blood transfusions, and chemotherapy drugs.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients