Central Precocious Puberty (CPP) is referred to as gonadotropin-dependent precocious puberty. It is an endocrine-related developmental disease characterized by the onset of pubertal changes with the development of secondary sexual characteristics, accelerated growth, and bone maturation before the normal age of puberty (8 years in girls and 9 years in boys).
This condition is idiopathic. However, the most common known genetic cause of central precocious puberty is mutation in the MKRN3 gene. In addition to genetics, other influential factors which may affect the condition, include nutrition, socioeconomic status, and exposure to certain chemicals in the environment.
Global Central Precocious Puberty Market - Impact of the Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared COVID-19 a pandemic on March 11, 2020. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, over 346.7 million cases and 5.5 million deaths due to Coronavirus (COVID-19) were reported till January 23, 2022, across the globe.
Impact of COVID-19 on Demand and Supply of Central Precocious Puberty Treatment Drugs
The COVID-19 pandemic and its consequent lockdown in various countries have impacted the financial status of businesses across all sectors including the private healthcare sector. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. The COVID-19 pandemic has also affected the economy of various regions across the globe in three main ways: 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; 3) through its financial impact on companies and financial markets. Several countries such as Thailand, Indonesia, and Singapore are facing problems with regard to transportation and distribution of healthcare products.
The impact of the COVID-19 pandemic is expected to limit the growth of the global central precocious puberty market during the forecast period, owing to a decrease in the supply of raw materials and pharmaceutical drugs of manufacturing companies associated with central precocious puberty.
The global central precocious puberty market is estimated to be valued at US$ 1,595.2 Mn in 2022 and expected to exhibit a CAGR of 7.9% over the forecast period (2022-2030).
Figure 1: Global Central Precocious Puberty Market Share (%) Analysis, By Drug, 2022
To learn more about this report, Download Free Sample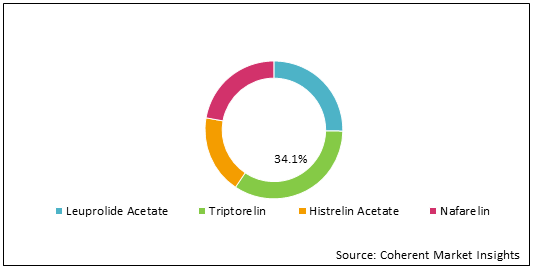
Increasing product launches and approvals for the treatment of central precocious puberty are expected to drive the market growth
Market players are focusing on obtaining product approval from regulatory authorities such as U.S. Food and Drug Administration (FDA), which is expected to augment the market growth over the forecast period. For instance, in May 2020, Tolmar Pharmaceuticals, Inc., a pharmaceutical company, received approval for its New Drug Application (NDA) from the U.S. FDA for FENSOLVI (leuprolide acetate) for injectable suspension for the treatment of pediatric patients aged two years and older with central precocious puberty.
Central Precocious Puberty Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2022: | US$ 1,595.2 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2022 to 2028 |
| Forecast Period 2022 to 2030 CAGR: | 7.9% | 2030 Value Projection: | US$ 2,926.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceutical Industries Ltd., AbbVie Inc., Arbor Pharmaceuticals, LLC, Pfizer Inc., Tolmar Pharmaceuticals, Inc., Endo International plc, Ipsen Pharma, Debiopharm Group, and Sun Pharmaceutical Industries Limited |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing partnerships and agreements among market players are expected to augment the market growth
Market players are focusing on various inorganic strategies such as partnerships and agreements, which is anticipated to propel the global central precocious puberty market growth over the forecast period. For instance, in January 2016, Debiopharm International SA (Debiopharm), part of Debiopharm Group, entered into an exclusive distribution agreement with Arbor Pharmaceuticals, a pharmaceutical company, for the commercialization and promotion of triptorelin 22.5 mg for CPP in the U.S.
Global Central Precocious Puberty Market – Restraints
Side effects associated with drugs such as Triptodur are anticipated to hinder the marker growth over the forecast period. Side effects associated with Central Precocious Puberty treatment drugs are painful or difficult urination, burning while urination, blood in the urine, bone pain, (in children) new or worsening signs of puberty, seizure, chest pain or pressure, pain spreading to jaw or shoulder, sudden numbness or weakness, and slurred speech.
Global Central Precocious Puberty Market – Regional Analysis
On the basis of region, the global central precocious puberty market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to hold a dominant position in the global central precocious puberty market over the forecast period, owing to the increasing availability of drugs for the treatment of central precocious puberty in the U.S. For instance, in October 2017, Arbor Pharmaceuticals, LLC and Debiopharm International SA announced the commercial availability of Triptodur in the U.S. for the treatment of pediatric patients aged two years and older diagnosed with central precocious puberty (CPP).
Figure 2: Global Central Precocious Puberty Market Value (US$ Mn), by Region, 2022
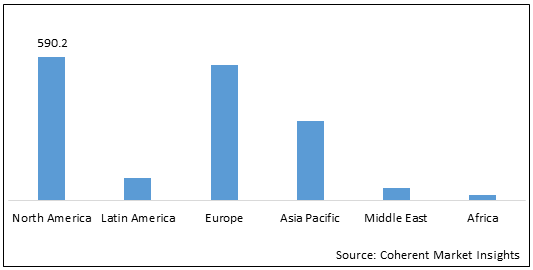
To learn more about this report, Download Free Sample
Global Central Precocious Puberty Market – Competitive Landscape
Major players operating in the global central precocious puberty market include Teva Pharmaceutical Industries Ltd., AbbVie Inc., Arbor Pharmaceuticals, LLC, Pfizer Inc., Tolmar Pharmaceuticals, Inc., Endo International plc, Ipsen Pharma, Debiopharm Group, and Sun Pharmaceutical Industries Limited.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients