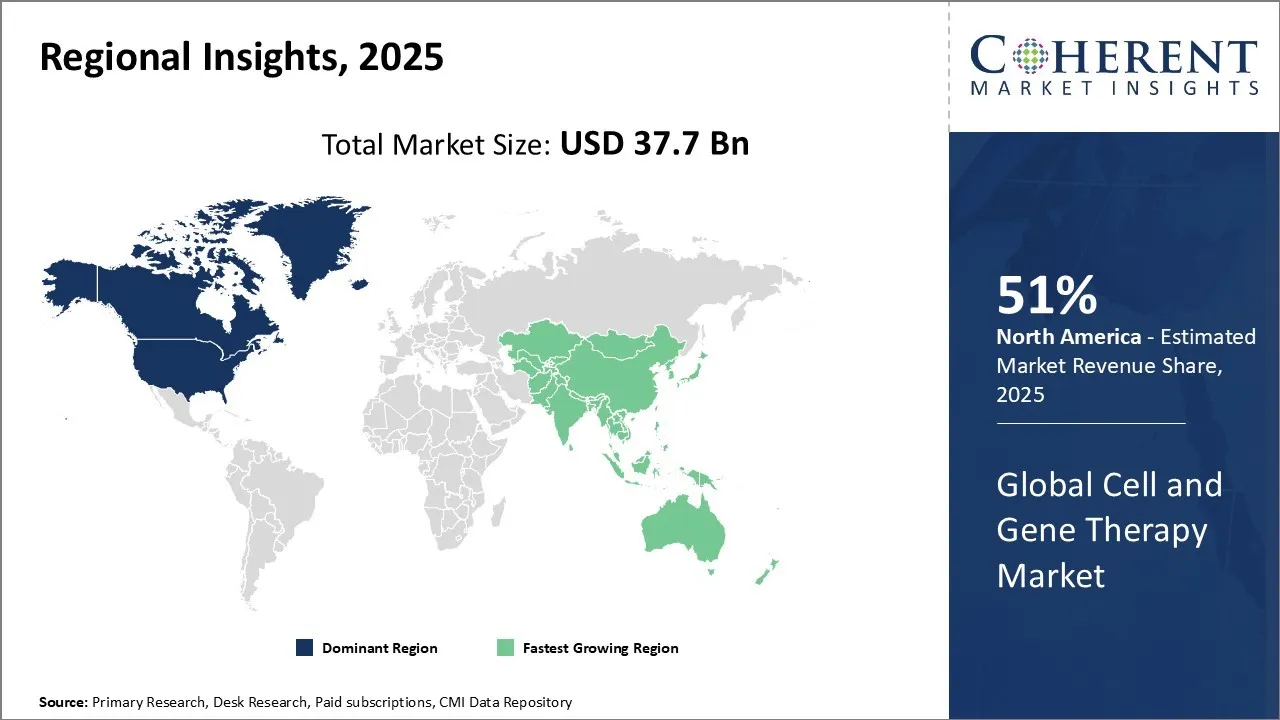
The global cell and gene therapy market is estimated to be valued at USD 37.7 Bn in 2025 and is expected to reach USD 125.5 Bn in 2032 exhibiting a CAGR of 18.5% during the forecast period (2025-2032).
The global cell and gene therapy market demand is primarily driven by curative, targeted, and highly personalized solutions for both common and rare diseases. CGTs target the root genetic or cellular causes of diseases, thus is the high prefers of it. There are over 7,000 rare diseases, most of which are genetic and lack effective treatments, where GT offers targeted solutions through gene correction, replacement, or cell-based regeneration, driving significant demand in orphan drug markets. The demand is further fueled by a broader shift toward personalized, precision medicine and regenerative solutions, particularly in regions like the U.S., Europe, China, and South Korea.
|
Current Event |
Description and its Impact |
|
Regulatory Policy Shifts |
|
|
Investment & Market Dynamics |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The integration of artificial intelligence (AI) into cell and gene therapy market is significantly influencing by accelerating discovery, optimizing manufacturing, personalizing treatment and improving clinical outcomes. AI helps scientists to identify and optimize therapeutic targets by analyzing vast range of data sets, including genomic, proteomic, and cellular interaction data. For instance, AI algorithms were used to design more efficient CAR-T constructs, optimize gene editing targets (e.g., for CRISPR), and predict off-target effects. AI also helps in matching patients with a suitable therapy by integrating genomic, phenotypic, and clinical data.
In June 2025, Stanford researchers harnessed machine learning to enhance the safety and effectiveness of cell and gene therapies. The team developed a trio of AI models that simultaneously design functional therapeutic proteins and predict immune system reactions, enabling the creation of “de‑immunized” proteins with minimal rejection risk.
Since 2019, Japan has approved five CAR-T cell therapies and four gene therapies, all receiving public reimbursement post-launch. CGTs in Japan are reimbursed either as pharmaceuticals or medical devices, based on their mechanism of action and intended use.
For pharmaceutical CGTs, pricing is determined through either cost-based or comparator-based methods, with potential premiums from nine designated categories. Kymriah, the first CAR-T therapy, was priced using the cost-based method and received premiums for orphan status, novel mechanism, and efficacy. Subsequent CAR-T therapies used Kymriah as a pricing benchmark. Zolgensma, priced via the comparator method using Spinraza, earned 10% Senkuteki and 50% Usefulness (I) premiums.
Four months after launching the Cell & Gene Therapy Access Model for Medicaid, CMS proposed an enhancement to the New Technology Add-on Payment (NTAP) for Medicare beneficiaries. Under the proposed change, Medicare would reimburse 75% of the cost of qualifying gene therapies—an increase from the current 65% reimbursement rate.
This proposed update is critical for high-cost gene therapies such as:
Both therapies received FDA approval in December 2023, marking a milestone in gene editing and gene addition technologies for sickle cell disease.
In terms of therapy type, the cell therapy segment is expected to dominate the global cell and gene therapy market with a share of 88.5% in 2025, and this is due to the increasing adoption of various growth strategies such as collaboration, funding, and others by the market players. cell therapies are often patient-specific, offering precise targeting with fewer side effects, which is especially important in oncology, where precision is key to sparing healthy tissue while attacking cancer cells.
For instance, Chimeric antigen receptor T (CAR‑T) cell therapy is generating fresh optimism in oncology, moving beyond blood cancers into solid tumors. At the 2025 ASCO conference in Chicago, research showed CAR‑T treatments extended survival by 40% in gastric and gastroesophageal junction cancers, with tumor shrinkage observed in recurrent glioblastoma cases. Additionally, cell therapy is advancing in manufacturing and scalability through technological developments in cell culture, automation, and cryopreservation are making cell therapies more scalable and affordable. Companies are investing heavily in GMP-compliant facilities and point-of-care delivery models, improving accessibility and turnaround times.
In May 2025, a new Cell Development Organization (CDO), Minaris Advanced Therapies, launched to address manufacturing and development bottlenecks in the regenerative medicine industry. The CDO offers end-to-end solutions spanning process development, scale-up, quality control, and regulatory compliance for cell and gene therapy developers.
In terms of application, the musculoskeletal segment is expected to dominate the global cell and gene therapy market during the forecast period, and this is due to the increasing prevalence of musculoskeletal injuries such as cartilage defects, osteoarthritis, ligament, and others. Musculoskeletal conditions often involved tissue that heals poorly or not at all, where cell therapy using mesenchymal stem cells (MSCs), has shown promising regenerating cartilage, reduced inflammation and improving joint function. Cell therapy also helps in addressing chronic inflammation and pain, CGTs modulate immune responses and inflammation at the molecular level.
In March 2025, clinical trial results published by Medipost reveal that its stem cell therapy Cartistem significantly outperforms traditional microdrilling methods in regenerating cartilage for patients with knee joint defects. The study showed that treated patients regained cartilage strength and resilience at levels nearly matching healthy tissue, while the microdrilling control group exhibited inferior repair quality.
In terms of end user, the hospitals segment is expected to dominate the market over the forecast period, and this is due to the collaborations by hospitals with the market players to support clinical trials and research and development activities of cell and gene therapy. Hospitals are becoming a central to the delivery of CGTs as these therapies often requires specialized infrastructure, multidisciplinary teams, and complex patient management protocols. Many governments are launching hospital-based reimbursement models for CGT, such as outcomes-based payments or installment-based pricing. Some hospitals have begun building GMP-compliant clean rooms or partnering with CGT companies to enable point-of-care manufacturing for faster and cost-effective delivery.
In June 2025, researchers at St. Jude Children’s Research Hospital reported that a novel gene therapy significantly improves cerebral blood flow in adults with sickle cell disease, potentially reducing stroke risk. In a clinical trial involving three patients, magnetic resonance imaging revealed a 22– 43% decrease in elevated brain blood flow speeds, restoring values to normal and sustaining the improvement over one to two years.
To learn more about this report, Download Free Sample
North America is expected to dominate the global market with 51% share in 2025, due to increasing funding by the market players in cell and gene therapy under this region. North America has the rising number of rare diseases which significantly drives the market demand for cell and gene therapy. According to Penn Medicine, there are currently 5 FDA-approved gene therapy treatments available in the United States. Some popular centres include Boston Children’s Hospital Gene Therapy Program, Cell and Gene Therapy collaborative at CHOP, Cell and Gene Therapy center at RowanSOm/Cooper Hospital and many more.
In June 2025, the ProBio, a global contract development and manufacturing organization (CDMO) specializing in cell and gene therapy opened its flagship Cell and Gene therapy Center of Excellence at the Princeton West Innovation Campus in Hopewell, New Jersey. The premise facility includes high-quality in plasmid DNA and Viral vectors including adeno-associated virus (AAV) and lentiviral vector (LVV) platforms, reflecting ProBio's dedication to accelerating the delivery of transformative medicines.
The Asia Pacific cell and gene therapy market is anticipated to be the fastest growing region during the forecast period. The growth is Asia Pacific region is fueled by significant investment, advanced research, and clinical development of infrastructure. The investment into cell and gene therapy is focused on drug modalities, to provide better safety, manufacturing and scale-up. For instance, in November 2023, AtraZeneca announced a collaboration and investment agreement with Cellectis, a clinical-stage biotechnology company, to accelerate the development for next generation therapeutics in areas of high unmet needs including oncology, immunology and rare diseases. This is further proliferating the cell and gene therapy market demand.
The United States remains at the forefront of innovative research, especially in the field of CAR-T therapies that aim to tackle a broader spectrum of cancers, including solid tumors. Although navigating the regulatory landscape here can be challenging, the robust biotech ecosystem and access to cutting-edge technologies pave the way for continued advancements in cell and gene therapy research.
In November 2024, the University of Texas MD Anderson Cancer Center launched its Institute for Cell Therapy Discovery & Innovation. This initiative is set to leverage MD Anderson's extensive clinical and research experience to lead global advancements in impactful cell therapies for those who need them most. The institute will unite leading scientists and clinicians to pursue groundbreaking research across discovery, translational, and clinical domains. This collaboration aims to provide fresh insights into immunology and cell engineering, paving the way for innovative treatments that can swiftly adapt to emerging challenges in cancer, autoimmune diseases, infections, and more. So far, the efforts have been significantly supported by philanthropic contributions and institutional backing exceeding USD 80 million.
Japan continues to lead the Asia Pacific cell and gene therapy market growth with large development of cell and gene therapies. Japan became the first country to approve a cell therapy product, a treatment for macular degeneration. The country has approved several other cell and gene therapies, including a CAR-T therapy for leukemia and lymphoma. Japan has the presence of several companies and research institutions working on innovative treatments. Some of the key players in Japan include Fujifilm Cellular Dynamics, Astellas Pharma, and Takeda Pharmaceutical.
In June 2025, AGC Biologics, introduced cell therapy process development and clinical manufacturing services at AGC Inc.’s Yokohama Technical Center, marking the latest step in the global expansion of the company’s Global Cell and Gene Technologies Division. The improved geographical footprint allows AGC Biologics to better serve customers requiring autologous and allogeneic products across all markets, with cell therapy manufacturing now available in three continents (Milan, Italy – Longmont, Colorado, U.S. – Yokohama, Japan). This is further accelerating the cell and gene therapy market revenue.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 37.7 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 18.5% | 2032 Value Projection: | USD 125.5 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, Pfizer, Inc., Sanofi S.A., Amgen, Inc., Regeneron Pharmaceuticals, Inc., Bluebird Bio, Inc. (Celgene Corporation), Biogen Inc., uniQure N.V., JCR Pharmaceuticals Co. Ltd., Gene Biotherapeutics, Kolon TissueGene, Inc., Horama S.A., MeiraGTx Limited, Gilead Sciences, Inc., Organogenesis, Inc., Orchard Therapeutics Plc., Freeline Therapeutics Ltd., Bristol-Myers Squibb Company, PTC Therapeutics, Inc., Spark Therapeutics, Inc., and Biomarin Pharmaceutical Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The key market players are focused on making collaborations with other companies to develop new gene therapies and commercialize those in the cell and gene therapy market. For instance, in February 2023, Charles River Laboratories International, Inc., a pharmaceutical company, and Purespring Therapeutics, a pioneering gene therapy company focused on transforming the treatment of kidney diseases, announced a plasmid DNA contract development and manufacturing organization (CDMO) collaboration. Under the collaboration, the gene therapy platform targeting renal diseases, using Charles River’s established plasmid platform, eXpDNA, and decades of experience at the company’s plasmid DNA manufacturing center of excellence.
The rising focus on cell and gene therapy market research is fueling innovation for several challenging diseases. Recent breakthroughs include FDA-approved gene therapies for hemophilia B: Fidanacogene elaparvovec (Beqvez), which showed a 71% reduction in bleeding episodes in Phase III trials and sustained benefits up to six years, and Etranacogene dezaparvovec (Hemgenix), demonstrated sustained Factor IX activity and no serious adverse events at three years post-treatment.
Meanwhile, Huntington’s disease is advancing through gene therapy research: uniQure’s AMT‑130 received FDA RMAT designation and an FDA testing agreement in late 2024, opening paths to accelerated approval if efficacy is confirmed. These milestones illustrate accelerated progress in developing regenerative treatments for both rare genetic and neurodegenerative disorders.
Such research advances are driving broader adoption and commercialization, signaling a growing role for CGT in managing complex diseases.
The cell and gene therapy market forecast continues to strengthen as leading players increasingly embrace inorganic growth strategies such as acquisitions, equity investments, and strategic partnerships. This trend accelerates innovation, expands production capacity, and enriches clinical pipelines.
In July 2025, Pfizer completed its acquisition of FabCyte, a clinical-stage gene therapy startup focused on Duchenne muscular dystrophy, for USD 1.2 Bn, instantly broadening its gene therapy footprint. Similarly, Bristol Myers Squibb secured a $600 million upfront deal with Mammoth Biosciences in June 2025 to co-develop CRISPR-based therapies for rare genetic disorders.
On the manufacturing side, Thermo Fisher Scientific acquired GenCell Bio in late 2024, adding a state-of-the-art viral vector production facility in Europe. Meanwhile, Novartis expanded its U.S. manufacturing by acquiring Cellura Bioproduction in early 2025.
These moves combine cutting-edge biotech with manufacturing scale are effectively compressing development timelines and reducing costs, enhancing readiness for late-stage trials and commercialization, and shaping a robust cell and gene therapy market forecast for the coming decade.
The cell and gene therapy (CGT) market is entering a transformative phase, with future opportunities spanning across technology, disease areas, and global accessibility. One of the most significant possibilities lies in expanding CGT beyond hematologic cancers into solid tumors, where breakthroughs such as dual-targeted CAR-T cells and oncolytic viruses are showing early promise in lung, breast, and brain cancers. Another major shift is the move from autologous to off-the-shelf (allogeneic) therapies, which are expected to dramatically reduce cost and improve scalability, especially through CRISPR-engineered immune cells.
In parallel, there is strong momentum toward developing CGTs for neurological and autoimmune diseases, including ALS, multiple sclerosis, and lupus, using stem cells and regulatory T-cells. The emergence of advanced gene editing tools like prime and base editing also opens new therapeutic avenues beyond rare genetic conditions, targeting complex disorders such as cardiovascular disease and diabetes.
*Definition: Stem cell therapy (SCT) involves the treatment of various disorders ranging from non-serious to life-threatening diseases by using stem cells. Stem cells are used to potentially treat more than 80 disorders, including neuromuscular and degenerative disorders. Gene therapy is an approach to treat genetic diseases by augmenting, replacing, or suppressing the mutated genes with functional copies. They address the root cause of an inherited disease by enabling the body to produce necessary proteins and restore normal functioning. Gene therapy involves the transfer of genetic material through a suitable carrier or vector.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients