Catheter Related Blood Stream Infection Market Size and Forecast – 2025 – 2032
The Global Catheter Related Blood Stream Infection Market size is estimated to be valued at USD 3.8 billion in 2025 and is expected to reach USD 6.5 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.1% from 2025 to 2032.
Global Catheter Related Blood Stream Infection Market Overview
Catheter-Related Bloodstream Infections (CRBSIs) occur when bacteria or fungi enter the bloodstream through a catheter, typically used for administering medication, fluids, or nutrition. They are one of the most common and serious complications associated with intravascular devices, especially central venous catheters. CRBSIs can lead to severe illness, prolonged hospital stays, and increased healthcare costs. They are often diagnosed based on clinical symptoms such as fever, chills, or hypotension, and confirmed through positive blood cultures. Certain patient populations, such as those in critical care, undergoing dialysis, or receiving chemotherapy, are at higher risk due to frequent catheter use and compromised immunity.
The market for CRBSI prevention and management is driven by the growing prevalence of chronic diseases requiring long-term vascular access, rising hospital admissions, and heightened awareness of infection control. Advances in catheter materials, antimicrobial coatings, and closed-system technologies are reducing infection rates. Guidelines from health organizations emphasize proper hand hygiene, catheter site care, and timely catheter removal. Despite progress, CRBSIs remain a major challenge, especially in developing healthcare settings with limited resources. Continued innovation in device design and adherence to preventive protocols are essential to improving patient outcomes and reducing the global burden of these infections.
Key Takeaways
In the Catheter types segment, CVCs lead due to long-term use in critical care and oncology, supported by antimicrobial innovations. PVCs grow fastest driven by short-term outpatient needs. Arterial and specialized catheters fill niche monitoring roles.
In the application segment, Dialysis drives market dominance due to chronic kidney disease trends. Infusion Therapy grows rapidly with outpatient biologic use. ICU monitoring remains vital, while Chemotherapy and emerging home care applications contribute steadily.
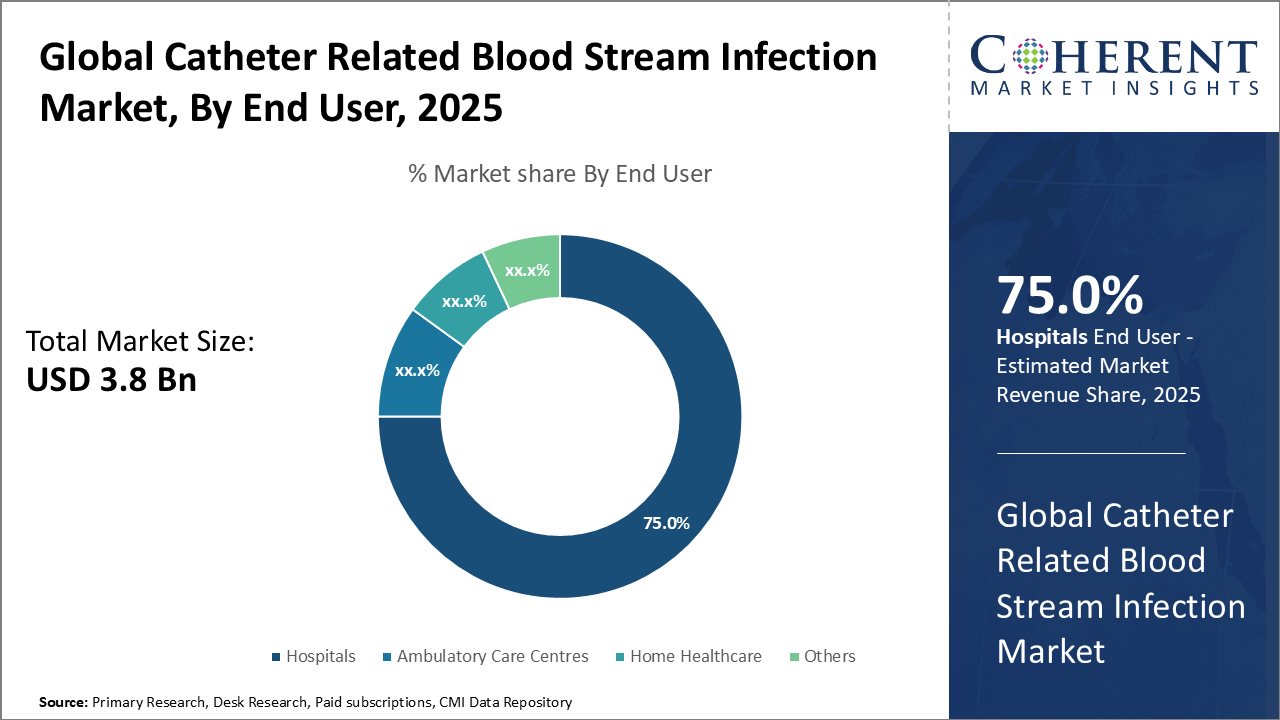
In the end user segment, Hospitals dominate with 75% share via high catheter usage and compliant infection protocols. Ambulatory Care Centres grow fastest with outpatient trends. Home Healthcare expands for chronic care, while specialty clinics add niche demand.
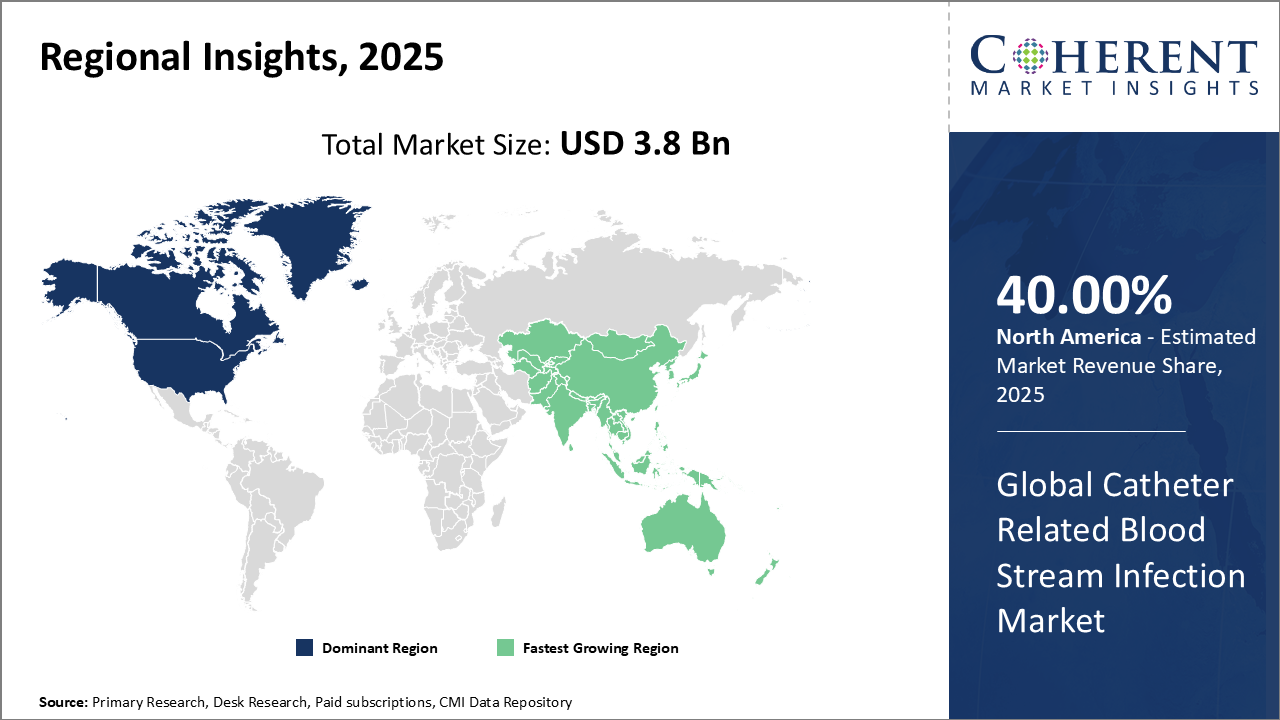
North America dominates with 40% market share, driven by advanced infrastructure and CMS-backed antimicrobial adoption. Asia Pacific leads growth with 9.2% CAGR underpinned by expanding healthcare access and chronic disease burden. The U.S. advances infection control via smart catheter usage, regulatory oversight, and strong R&D.
Catheter Related Blood Stream Infection Market Segmentation Analysis
To learn more about this report, Download Free Sample
Catheter Related Blood Stream Infection Market Insights, By End User
Hospitals dominate the market with approximately 75% revenue share, driven by extensive catheter use in ICUs, oncology units, and dialysis centres. Their investment in advanced infection control products is supported by regulatory standards and reimbursement policies favouring antimicrobial catheter technologies. Ambulatory Care Centres are the fastest-growing segment due to the shift toward outpatient and minimally invasive procedures, increasing demand for safe, easy-to-use catheters. Home Healthcare is gaining importance as chronic disease management moves to home settings, requiring patient-friendly devices. “Others” include specialty clinics and long-term care facilities.
Catheter Related Blood Stream Infection Market Insights, By Catheter Type
In the catheter type segment, Central Venous Catheters (CVCs) dominate the CRBSI market due to their essential role in long-term access for intensive care, dialysis, and oncology, supported by advancements in antimicrobial coatings. Peripheral Venous Catheters (PVCs) are the fastest-growing segment as they are widely used in outpatient care and emergency settings for short-term treatments, offering easy insertion and cost benefits. Arterial catheters play a moderate role in continuous blood pressure monitoring, while “Others” include specialized catheters for niche clinical uses.
Catheter Related Blood Stream Infection Market Insights, By Application
Dialysis dominates the market due to the rising prevalence of chronic kidney disease requiring long-term catheter use and improved biocompatible designs. Infusion Therapy is the fastest-growing segment, driven by outpatient care expansion and increased use of biologics and parenteral nutrition. Monitoring applications are critical in ICU settings for continuous data. Chemotherapy contributes moderately, while “Others” include emerging uses such as home-based care, supporting incremental market growth.
Catheter Related Blood Stream Infection Market Trends
The market is shifting toward closed-system catheter maintenance, improving safety and reducing contamination during handling.
Sensor-embedded catheters are gaining traction, enabling real-time infection monitoring, with European ICU pilot programs reporting a 20% reduction in CRBSI cases in 2024.
Digital health and telemedicine are increasingly used for remote monitoring post-catheter insertion, enhancing patient care beyond hospital settings.
Biodegradable and sustainable catheter materials are emerging, driven by a 15% annual rise in adoption across Asia Pacific in 2024, reflecting growing environmental responsibility.
These innovations highlight a combined focus on infection reduction, patient safety, and eco-conscious solutions shaping the future of catheter care.
Catheter Related Blood Stream Infection Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Catheter Related Blood Stream Infection Market Analysis and Trends
North America is the dominating and steadily growing region in the CRBSI market, driven by advanced healthcare infrastructure, comprehensive infection prevention protocols, and favourable reimbursement frameworks. In the U.S., the implementation of CMS policies tied to infection metrics has accelerated the adoption of antimicrobial catheter technologies. The region accounts for roughly 40% of global market share, with key companies like Becton Dickinson and 3M leading innovation and expanding product portfolios to meet rising demand.
Asia Pacific Catheter Related Blood Stream Infection Market Analysis and Trends
Asia Pacific is the fastest-growing region in the market, expanding at a CAGR of over 9.2%. This growth is driven by rapidly improving healthcare infrastructure, increased government investment in acute care services, and a rising burden of chronic diseases requiring catheter use. The region also benefits from the growing presence of multinational players like Terumo and Teleflex, paired with a strong focus on cost-effective infection prevention. Key markets such as China, India, and Southeast Asia are at the forefront of this expansion.
Catheter Related Blood Stream Infection Market Outlook for Key Countries
USA Catheter Related Blood Stream Infection Market Analysis and Trends
The USA remains a dominating market due to strict regulatory oversight, high adoption of antimicrobial and smart catheters, and strong investment from both public and private healthcare sectors. In 2024, over 70% of ICU catheter insertions utilized antimicrobial agents, helping achieve a 10% year-on-year reduction in bloodstream infections, as per CDC data. Key manufacturers like Becton Dickinson and Smiths Medical are leading innovation through heavy R&D spending and integrated infection prevention programs. Additionally, value-based care initiatives by the U.S. government continue to support the widespread use of advanced catheter technologies.
Analyst Opinion
The rising adoption of antimicrobial-impregnated and antiseptic-coated catheters is a key supply-side factor boosting market expansion. In 2024, hospital procurement of coated catheters increased by 12% compared to 2023, with studies showing up to a 40% reduction in infection rates when these products are used.
On the demand side, catheter usage surged in oncology and dialysis due to the rising prevalence of chronic kidney disease, which grew by 9% globally in 2024. Hospitals also reported a 15% increase in ICU catheter use, reflecting growing demand for infection prevention solutions.
Pricing pressures continue to shape market dynamics, with average catheter costs rising by around 4.3% in 2025 due to advanced features and compliance requirements. In North America, reimbursement reforms are encouraging the use of infection-reducing technologies, influencing hospital purchasing behaviours.
Micro-indicators like hospital-acquired infection monitoring and stricter reporting standards are also driving market growth. In the U.S., CMS penalties for high CRBSI rates led to a 10% decline in infection cases from 2023 to 2024, highlighting the impact of improved risk management and accountability measures.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 3.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: |
USD 6.5 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Becton Dickinson and Company, 3M Company, Smiths Medical, Teleflex Incorporated, Abbott Laboratories, ICU Medical, Inc., Bard Access Systems, C. R. Bard Inc., AngioDynamics Inc., Baxter International Inc., Terumo Corporation, Cook Medical, Merit Medical Systems Inc., Nipro Corporation, Cardinal Health, Inc., B. Braun Melsungen AG, Nanomedic Technologies, Medtronic PLC, Guerbet Group, Fresenius Medical Care | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Catheter Related Blood Stream Infection Market Growth Factors
Catheter-Related Bloodstream Infection (CRBSI) market growth is driven by heightened awareness of hospital-acquired infections and stricter regulatory frameworks mandating infection monitoring and reporting. The FDA’s updated 2023 guidelines on vascular access devices have accelerated the adoption of safer, next-generation catheter solutions. Rising prevalence of chronic conditions such as cancer and renal failure, which often require prolonged catheter use, is also significantly increasing market demand.
Technological advancements in antimicrobial and antiseptic-coated catheters are strengthening infection prevention efforts, with clinical trials in 2024 showing a 35% improvement in efficacy compared to standard catheters. Additionally, the expansion of home healthcare services is fuelling growth, as more patients opt for outpatient and home-based care for convenience and cost-effectiveness. This trend is especially notable in developed regions like North America and Europe.
Catheter Related Blood Stream Infection Market Development
In June 2025, scientists from Washington State University and the Mayo Clinic created an electrochemical catheter hub that could eventually help stop central line–associated bloodstream infections (CLABSIs), which kill thousands of people worldwide each year.
Key Players
Leading Companies of the Market
Becton Dickinson and Company
Smiths Medical
Teleflex Incorporated
Abbott Laboratories
ICU Medical, Inc.
Bard Access Systems
C. R. Bard, Inc.
AngioDynamics Inc.
Baxter International Inc.
Terumo Corporation
Cook Medical
Merit Medical Systems Inc.
Nipro Corporation
Cardinal Health Inc.
B. Braun Melsungen AG
Nanomedic Technologies
Medtronic PLC, Guerbet Group
Fresenius Medical Care
Several market players have adopted differentiation strategies focusing on innovative antimicrobial coatings and integration of smart catheter technologies. For instance, Becton Dickinson launched a new line of chlorhexidine-impregnated catheters, resulting in a 25% increase in market share in 2024 in North America. Teleflex's strategic partnership with biotechnology firms to develop nanocoating solutions has enabled it to capture emerging market segments, contributing to a 30% growth in infusion therapy applications in Asia Pacific markets.
Catheter Related Blood Stream Infection Market Future Outlook
The future outlook for the Catheter-Related Bloodstream Infection (CRBSI) market remains highly positive, driven by continuous advancements in catheter design, materials, and infection prevention technologies. The adoption of antimicrobial and antiseptic-coated catheters, along with smart sensor-enabled systems for real-time infection monitoring, is expected to significantly reduce CRBSI incidence and improve patient safety. Increasing emphasis on value-based healthcare and regulatory support for infection control will continue to drive investments in safer catheter solutions. Expansion of home healthcare and telemedicine platforms is transforming post-catheter insertion care, enabling remote monitoring and early infection detection. Moreover, the industry is witnessing rising interest in biodegradable and eco-friendly catheter materials, aligning with sustainability goals. Continued innovation and growing demand for long-term vascular access in chronic disease treatments are set to shape the market's evolution.
Catheter Related Blood Stream Infection Market Historical Analysis
The Catheter-Related Bloodstream Infection (CRBSI) market has evolved significantly over recent decades, shaped by growing awareness of hospital-acquired infections and increased use of intravascular catheters in critical care, dialysis, and oncology. In the early 2000s, high infection rates prompted the development of infection control guidelines and surveillance programs led by organizations like the CDC and WHO. Regulatory frameworks gradually pushed healthcare facilities to adopt stricter protocols and standardized sterile procedures. By the 2010s, the introduction of antimicrobial and antiseptic-coated catheters marked a major technological advancement, leading to measurable reductions in CRBSI cases. Innovations in catheter materials, insertion techniques, and maintenance practices, coupled with mandatory reporting and reimbursement policies, helped further drive down infection rates. Overall, the focus shifted from reactive infection treatment to proactive prevention.
Primary Research Interviews:
Infectious disease specialists
ICU and critical care nurses
Vascular access device manufacturers
Hospital infection control managers
Databases:
CDC – Healthcare-Associated Infection (HAI) Data
WHO – Global Infection Prevention and Control Reports
PubMed – Clinical Studies on CRBSI Prevention
Magazines:
Infection Control Today
Healthcare Hygiene Magazine
Medical Device and Diagnostic Industry (MD+DI)
Clinical Services Journal
Journals:
Journal of Hospital Infection
American Journal of Infection Control
Infection Control & Hospital Epidemiology
Journal of Vascular Access
Associations:
Association for Professionals in Infection Control and Epidemiology (APIC)
Society for Healthcare Epidemiology of America (SHEA)
Infusion Nurses Society (INS)
Infectious Diseases Society of America (IDSA)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients