Global castrate resistant prostate cancer market is estimated to be valued at USD 10.66 Bn in 2025 and is expected to reach USD 19.26 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
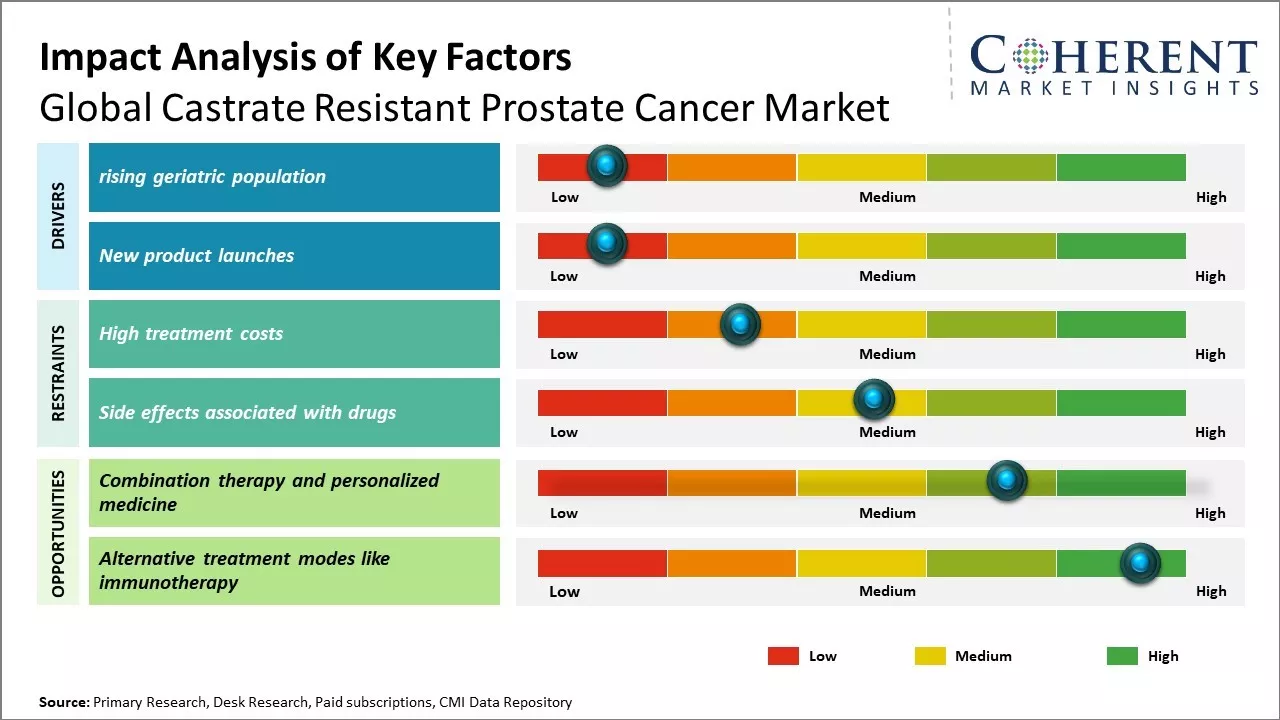
The market growth is primarily driven by rising geriatric population globally susceptible to develop prostate cancer and high adoption of premium-priced targeted therapies for metastatic castrate resistant prostate cancer.
Global castrate resistant prostate cancer market trend involves exponential rise in therapeutics and treatment options for patients with mCRPC. Introduction of new drug classes such as androgen receptor inhibitors, immune-modulators, kinase inhibitors along with combination therapies can transform the treatment algorithm and clinical outcomes in the near future. Wider acceptance of novel targeted therapies can drive the market growth during forecast period.
Rising Geriatric Population
Rising global population of elderly citizens aged 65 years and above can drive the castrate resistant prostate cancer market growth globally. According to the data published by World Health Organization in 2022, the number of people aged 65 years or older is projected to increase from 703 million in 2019 to 1.5 billion in 2050 worldwide. Prostate cancer is one of the highly prevalent cancer types seen in men above 65 years of age. As men progress to older age groups, their chances of developing prostate cancer and its castrate resistant form also increases considerably. Aging leads to weakening of the immune system and other physiological changes in the body, which make elderly men more vulnerable to cancers. Specifically for prostate cancer, rising levels of male hormones like testosterone in aging bodies create a supportive environment for cancer cells to thrive. Since castrate resistant prostate cancer is an advanced stage of prostate cancer where the primary treatment of hormone therapy becomes ineffective, its patient pool directly draws from the increasing population of elderly prostate cancer patients. This demographic shift boosts demand for novel drugs and therapies targeted towards managing castrate resistant prostate cancer symptoms and progression.
Get actionable strategies to beat competition: Download Free Sample
New product launches
The global castrate resistant prostate cancer market is witnessing strong growth driven by continuous product launches in the recent past years. New therapeutic options are offering hopes to prostate cancer patients who no longer respond to conventional hormone therapies. In last two years, three new drugs have been approved by FDA which has expanded the treatment landscape for metastatic castrate resistant prostate cancer (mCRPC) patients. In April 2020, FDA approved novel immunotherapy drug 'Pembrolizumab' in combination with enzalutamide or abiraterone for mCRPC patients. This was a major breakthrough as it provide an immune based treatment option. In May 2021, FDA granted regular approval to 'Apalutamide' for mCRPC patients who are also undergoing androgen deprivation therapy. This is a second generation oral anti-androgen drug and offers potential benefits over existing anti-androgen therapy.More recently in December 2021, FDA approved 'Larotrectinib' for adult and pediatric patients with solid tumors that have a NTRK gene fusion without a known acquired resistance mutation. This drug offers hope to mCRPC patients whose cancer has certain NTRK gene alterations. According to American Cancer Society's Cancer Facts & Figures 2023 report, such targeted therapies play a significant role in improving survival for many cancer types including prostate cancer in recent years. These continuous new product approvals are fueling strong growth in global castrate resistant prostate cancer market.
Key Takeaways from Analyst:
Global castrate resistant prostate cancer market growth is driven by rising prevalence of prostate cancer worldwide. Advancements in targeted therapy and immunotherapies can drive the market growth as these provide better treatment options compared to traditional chemotherapies. The approval and commercialization of several new drugs to treat late-stage prostate cancer can drive the market growth. However, high treatment cost associated with newer drugs can hamper the market growth.
North America is expected to dominate the global castrate resistant prostate cancer market during the forecast period due to high healthcare expenditure and growing awareness about advanced treatment options in the region.. Europe is also expected to hold a substantial share of the global market, owing to increasing government funding for cancer research. However, Asia Pacific region is likely to witness the fastest growth due to rising healthcare infrastructure, growing medical tourism industry and expanding base of pharmaceutical companies in the region.
Biologic therapy segment is anticipated have the highest growth rate during the forecast period attributing to newer product approvals and significant advantages offered by biologics over chemotherapy. Growing focus of leading pharmaceutical companies on introduction of novel biologic drugs can drive the segment growth. Developed economies can dominate the market for castrate resistant prostate cancer.
Market Challenges: High treatment costs
High treatment costs can hamper the global castrate resistant prostate cancer market growth. Prostate cancer treatments, especially in advanced and metastatic stages, involve lengthy and expensive therapies that place a huge financial burden on patients. The costs of newer targeted therapies and immunotherapies that provide survival benefits have increased substantially in recent years. For instance , according to the data from the National Health Service in the U.K., the cost of a course of treatment with apalutamide, a newer targeted therapy, can exceed US$ 10,000 per month. Immunotherapies like immune checkpoint inhibitors nivolumab and pembrolizumab cost over US$ 11,000 per month, as per data from the Centers for Medicare and Medicaid Services in the U.S. The rising economic burden of advanced prostate cancer care is restricting wider access to novel, lifesaving medications for many low and middle-income patients. This has a negative influence on therapy uptake and adherence. A study published by Journal of Oncology Practice in 2022 evaluated financial toxicity and costs associated with prostate cancer care using a national U.S. claims database. It reported that out-of-pocket costs placed an unsustainable financial strain on over 25% of patients, with costs averaging US$ 4,000 annually. High costs led to discontinuation of therapies or electing for stoppages/delays in treatment in several such cases. With treatment costs posing severe hardships, many patients regardless of geography and economic status are often left with little choice but to opt for less expensive but potentially less effective standard of care options instead of breakthrough therapies.
Market Opportunities: Combination therapy and personalized medicine
Combination therapy and personalized medicine offer a significant opportunity to advance care for patients with castrate resistant prostate cancer (CRPC). Traditionally, treatment has focused only on slowing cancer growth with chemotherapy or hormone therapy. However, newer scientific insights into tumor genetics and disease pathways have enabled more targeted, individualized treatment approaches. Personalized medicine embraces the concept that patients should receive therapies tailored to their unique genetic and molecular tumor profiles. No two prostate cancers are exactly alike - these can have different vulnerabilities and resistance mechanisms depending on their specific genetic mutations and alterations. A personalized approach seeks to identify these differences and match patients to treatments optimized for their tumor's specific characteristics. This strategy has already begun to transform care for other hard-to-treat cancers. For CRPC, combination therapies offer patients multidimensional treatment against their cancer's unique weaknesses. Recent advances have identified subgroups of CRPC driven by distinct genomic alterations such as defects in DNA damage response or androgen receptor signaling. Combining drugs that block these specific cancer-driving pathways simultaneously offers the potential for deeper and more durable responses than single agents alone. Pilot studies exploring biomarker-matched targeted combinations have demonstrated early signs of success.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
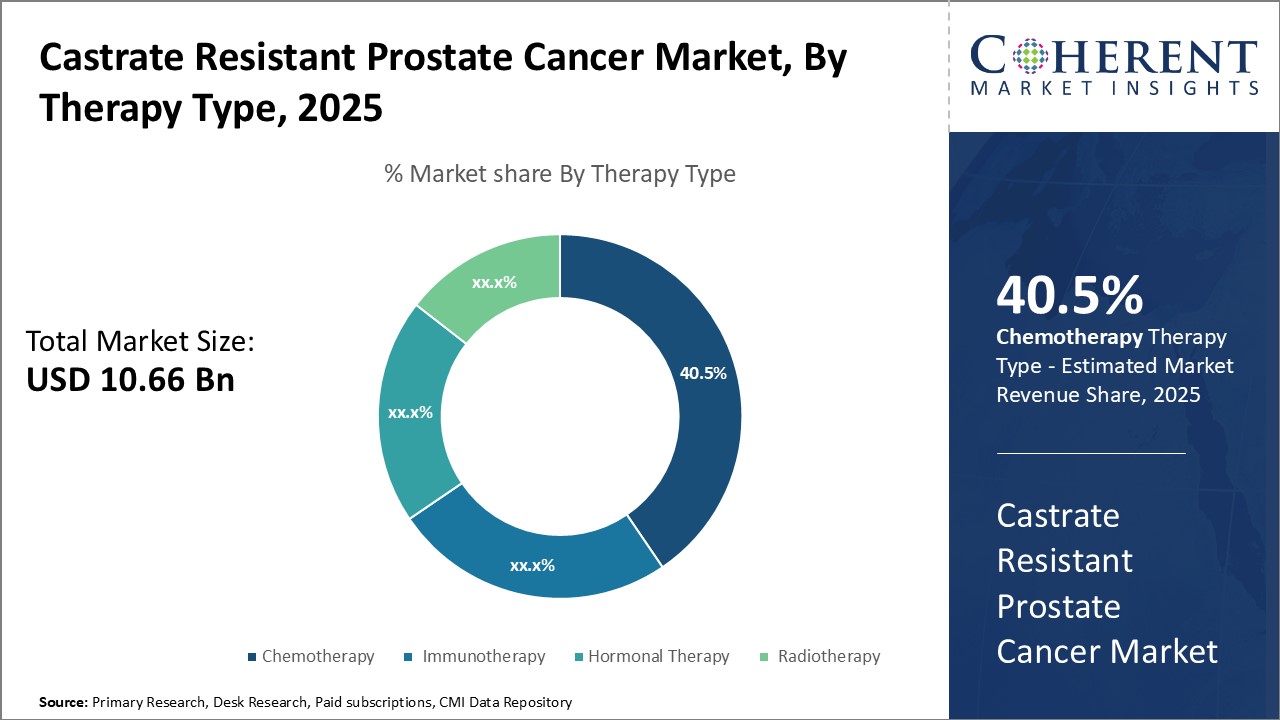
By Therapy Type- Advances in chemotherapy drugs can drive the chemotherapy segment growth
In terms of therapy type, chemotherapy segment is estimated to contribute the highest market share of 40.5% in 2025, owing to significant improvements in chemotherapy drugs for castrate resistant prostate cancer treatment. Newer chemotherapy drugs are more targeted and effective at treating metastasized cancer cells with reduced side effects. This has improved patient outcomes and quality of life. Continuous research into personalized chemotherapy can expand treatment options for varied patient conditions.
By Drug Class- Antineoplastic drugs remain standard-of-care
In terms of drug class, antineoplastic segment is estimated to contribute the highest market share of 35.62% in 2025, as these drugs are the standard first-line treatment. Docetaxel remains a core drug despite its side effects due to demonstrated efficacy benefits. Cabazitaxel is now commonly used after docetaxel therapy failure. Immunotherapy and other novel drug classes offer promise but often serve as adjuvant treatments or for later lines of therapy. Extensive clinical trials seek safer and more effective antineoplastic formulations.
By Route of Administration- Transition to oral medications
In terms of route of administration, oral segment is estimated to contribute the highest market share of 70.6% in 2025. This comes as new oral drugs enter the market, addressing patient preference for convenient oral dosing over injections. Oral medications allow for outpatient treatment and improve quality of life. However, injectable drugs still serve important roles where higher drug concentrations are needed or oral administration poses challenges.
Need a Different Region or Segment? Download Free Sample
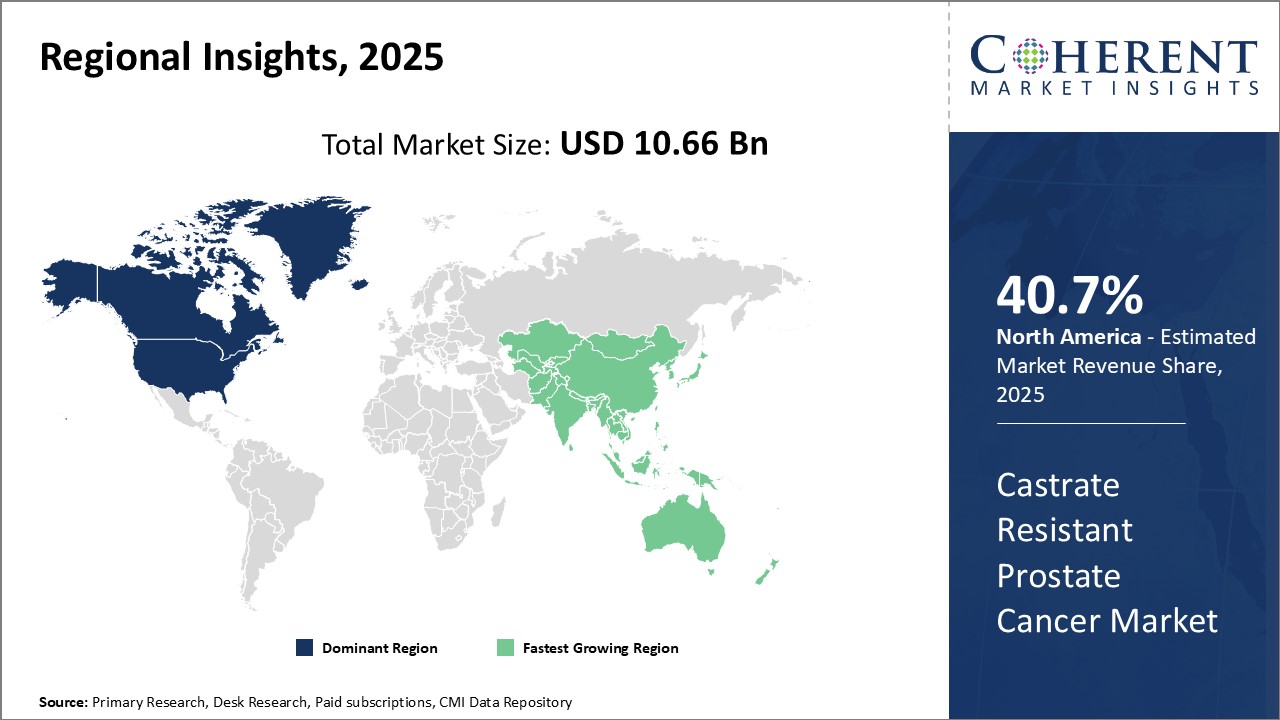
North America dominates the global castrate resistant prostate cancer market with an estimated market share of 40.7% in 2025. The U.S., being home to a large aging patient pool and high healthcare expenditure, contributes significantly to the regional market growth. Advanced diagnostic and treatment facilities in the U.S. and Canada have enabled effective management of late-stage prostate cancer. Leading pharmaceutical companies have established a strong foothold in these countries for development and commercialization of novel drug therapies. Support from government and non-profit organizations has also promoted research activities for castrate resistant prostate cancer.
Asia Pacific is expected to witness the fastest growth. Rapidly developing economies in the region like China and India are increasingly focusing on improving access to advanced cancer care. With rising healthcare budgets, greater acceptability of high-priced specialty medications and availability of generic drugs, treatment rates of castrate resistant prostate cancer will increase. Moreover, growing medical tourism industry attracts patients from developed markets in the West to the region for affordable treatment options. The region also offers lucrative business potential to international pharma firms to partner with local players and penetrate untapped rural markets. Collaborations between healthcare stakeholders will be crucial for spreading awareness on prostate cancer and augmenting diagnosis in Asia Pacific region.
Castrate Resistant Prostate Cancer Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 10.66 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.8% | 2032 Value Projection: | USD 19.26 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
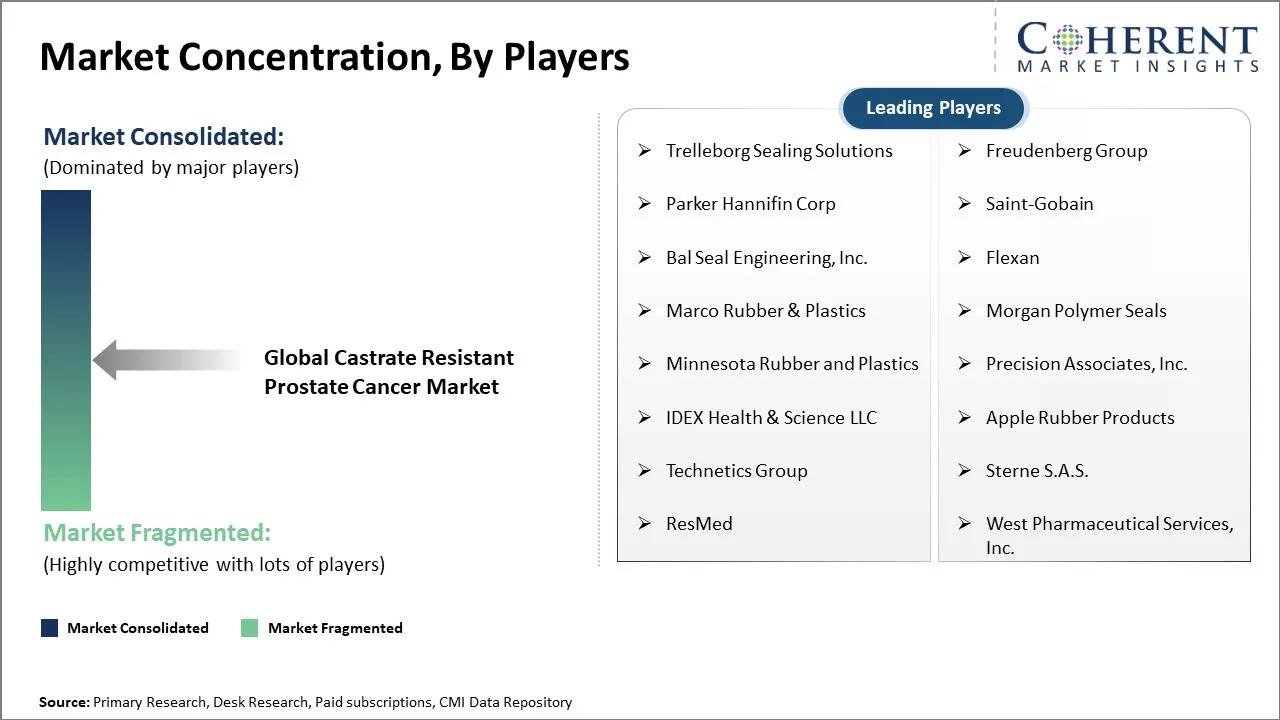
| Companies covered: |
Trelleborg Sealing Solutions, Freudenberg Group, Parker Hannifin Corp, Saint-Gobain, Bal Seal Engineering, Inc., Flexan, Marco Rubber & Plastics, Morgan Polymer Seals, Minnesota Rubber and Plastics, Precision Associates, Inc., IDEX Health & Science LLC, Apple Rubber Products, Technetics Group, Sterne S.A.S., ResMed, West Pharmaceutical Services, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global Castrate Resistant Prostate Cancer Market focuses on delivering treatment options for prostate cancer that has progressed after initial hormone treatment, known as castrate resistant prostate cancer (CRPC). This advanced stage of prostate cancer remains incurable with currently available therapies. The market involves companies developing new drugs, therapies, diagnostic tools and technologies to target CRPC and improve patient outcomes and survival rates. The goal is to advance treatment for this difficult-to-treat condition and enhance quality of life for those suffering from CRPC worldwide.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients