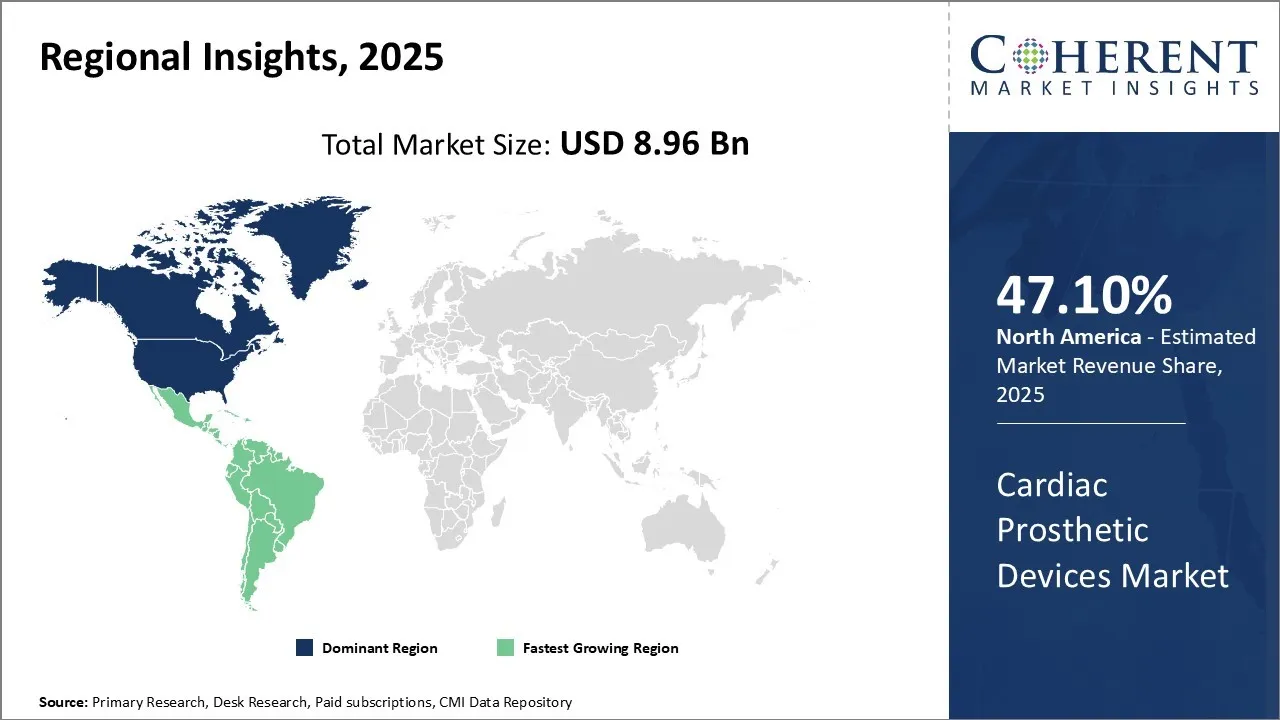
Cardiac Prosthetic Devices Market is estimated to be valued at USD 8.96 Bn in 2025 and is expected to reach USD 16.7 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Surgical processes increase significant demand for simple surgical processes, increasing aging population and increased burden of heart disease, demand for life -saving heartbeat solutions. These primary powers run the Global Cardiac Prosthesic Devices Market. These devices, such as heart valves and pacemakers, are designed to restore normal heart function, especially the patient, which makes them especially necessary for patients with heart failure, arrhythmia and valvular heart disease. His ability to reduce the risk of heart arresting, improve the quality of life and support minimal invasive heart surgery has established heart dates as significant intervention in both preventive and medical heart care.
Market expansion is largely affected by increasing investments in the health care system, increases the acceptance of advanced surgical technologies and supports reimbursement policy in developed and emerging economies. Parallel, awareness of early diagnosis, improvement of access to health care and lifestyle -related heart disease such as overweight and diabetes has increased, which accelerates by using heart protetic equipment globally. Innovations such as MRI-compatible pacemaker, Transcatator Aortic Valve Replacement (TAVR) and leadless pacemakers expand the patient's base by offering safe, less aggressive and long-term solutions.
For instance, In January 2025, Abbott Laboratories CE Mark approval for its Navitor ™ Transcatter Aortic Valve Implantation (TAVI) system, designed to treat patients with severe aortica loading stenosis who are at high surgical risk.
|
Current Event |
Description and its impact |
|
Global Push for Minimally Invasive Cardiac Procedures |
|
|
Investment Surge in MedTech and AI-Driven Cardiology |
|
|
Regulatory Approvals and Fast-Tracked Innovation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing incidence of heart disease such as atrial fibrillation and heart failure is estimated to run market growth during the forecast period. For example, in June 2022, a report published by the Atalata Alliance, AF Association and Stars - Patient Attorney Groups, Donations, Professional Medical Bodies and an alliance of industry interests - in 2021, in 2021, in 2021, 2021, the global spread of Alind Fibration reached around 37.574 million matters.
Leading market players require active approval to launch new products American FDA (Food and Drug Administration), and expects a significant contribution to the clause growth during the forecast period. In September 2021, for example, a leading medical tool manufacturer Abbott announced that the US FDA had approved its Epic Plus and Epic Plus Supra Stent tissue valve, improving the treatment options for people with aortic or mitral valve disease.
The increasing number of products published by the US FDA is estimated to prevent market growth during the forecast period. In May 2021, for example, Abbott (East known as St. Jude Medical), a medical equipment company, its absurdity and permanent pacemakers due to potential moisture, announced to reduce the electrical short -circuit and battery life. The American FDA classified it as a class, which I remember - the most serious type - reflects the risk of serious injury or death.
Further challenges related to the use of pacemaker will probably also interfere with market extension. For example, in May 2021, the United States FDA reported that some consumers may interfere with electronic equipment, such as some high -area mobile phones and magnets, with transplanted medical equipment. These can trigger the magnet pacemaker to enter magnetic mode, temporarily suspended the normal function of the magnet has moved away.
The market is classified in a heart valve and pacemaker. Of these, the pacemaker segment is estimated to lead the global market during the forecast period. This growth is mainly attributed to an increase in product approval from the US Food and Drug Administration (FDA), which is driven by active efforts from the players in the large market.
Depending on the latest user, the market is divided into hospitals, special clinics, ambulatory surgical centers and others. The hospital section is expected to have the largest market share during the forecast period, roughly due to the increasing global strain of heart disease.
To learn more about this report, Download Free Sample
North America is expected to maintain a prominent place in the global market for prognosis in the forecast. This dominance is mainly inspired by the increasing spread of heart failure in the region. For example, a report published by the Center for Disease Control and Prevention (CDC) stated January 5, 2025, that around 6.2 million adults in the United States lived with heart failure during 2018 to 2020 periods.
The United States is still a great strength in the global clothing march market, supported by a strong textile and fashion industry concentrated in cities such as New York and Los Angeles. A sharp-traditional urban lifestyle has driven a strong consumer change to practical and effective alternatives for traditional irons, such as clothing vapor. In addition, a well-installed retail infrastructure-two is widespread access to online and offline different steamer models. The presence of premium international and domestic brands, combined with consumer preferences for, practical equipment, continues to strengthen the management of the US market.
Canada plays an important role in the North American apparel Steamer market yet. User -friendly and increasing demand for hygiene home equipment is in line with quality -conscious Canadian consumers' preferences. When urban families use fast low-raving and smart solutions, clothing steamers get traction for their ability to refresh clothes without the need for a traditional ironing layout. The availability of extensive product through large retail chains and online platforms is presenting market growth across the country.
India emerges as a highly affected market for clothing steams, inspired by the rapidly expanded urban middle class and a well -established textile area. Increasing number of houses with two income and increasing employment in gender are creating a strong demand for time -saving, user -friendly home appliances. Government initiatives such as "Make in India" have encouraged both local and international brands to introduce cost -effective and functional steamer models. In addition, India's wide offline retail, including electronics and equipment stores, plays an important role in using the consumer.
Indonesia has established itself as a growing center in the apparel Steamer market in Southeast Asia. The expansion of a rich textile and clothing export industry as well as the population of the middle class, creating a stable demand for reliable clothing care tools. Urban consumers favor vapors for hygiene benefits, convenience and suitability for houses with limited location. Assistant government policy aimed at joint industrial development with active investment of local equipment brands in the expansion of retail, which further strengthens the position of Indonesia as a large emerging market.
Prices usually range from USD 5,000 to USD 20,000 per unit. Mechanical valves are high end because of their durability and require lifelong anticoagulation, while tissue valves are usually less expensive, but have a short life. The cost manufacturer varies by valve design and regulatory approval.
Price fixation for pacemakers is based on USD 2,500 to USD 10,000 to composite basis
Strict regulatory standards (eg FDA approval), due to advanced technology integration and high labor costs, prices are usually 20-30% higher than the global average. American pacemakers and heart valves may also include bundle prices for implantation and post -operative care, increase additional costs.
Countries such as India and China offer more financial prices, with pacemaker devices start from $ 1,500 and a heart valve to $ 3,000, roughly due to local production, state subsidy and medical tourism. However, imported equipment is still compared to western markets.
Pricing in Europe usually falls to a higher area in the middle, from $ 3,500 to USD 15,000, CE certification requirements, the health care policy and high product quality standards are affected. The reimbursement models and public health services affect price access to countries such as Germany, France and the United Kingdom.
The total procedure cost can range from $ 10,000 to USD 40,000, including resident of hospitals, surgeon taxes and anaesthesia. These costs often exceed the price of the unit, especially in the private health care system.
Pacemakers require regular check and battery replacements, usually every 5 to 10 years. The cost of replacement of batteries can range from 2000 to 5000 USD. Remote monitoring systems can also add running costs.
In case the product remembers, manufacturers can cover the costs of replacement of devices. However, surgical and hospital tax may apply until the insurance is covered by insurance.
Remote monitoring, MR compatibility and long battery wet devices are priced at Premium. For example, leadless pacemakers, their innovative design and minimum invasive implantation can cost 10,000 or more.
Reimbursement policies affect the final prices. In countries with public health coverage, patients often pay less than pockets, but prices are more for private institutions.
Advanced programming features, double -chambers sensing or integrated neuromodulator therapy can increase the prices of the unit by 10-25%, especially in specialized heart failure treatment models.
Special medical e-commerce platforms, safe, digital procurement platforms, hospitals and clinics, now source prosthetic heart valve pa arrangements (eg pacemaker and heart valves). These platforms streamline the ordering process, provide real -time storage updates, enable price comparison and provide wholesale discounts, especially useful for institutional buyers.
Manufacturers increase traditional distribution chains by selling health professionals directly through online channels. This model procurement leads the lead time and allows the unit for customization or production on request, thus improving accountability against the hospital's needs.
Platforms such as Facebook, Redit and Patient Platforms host groups dedicated to heart conditions, where users share experience with specific equipment, brands and results. These discussions affect the consumer's opinion and doctors dialogs on the unit's preferences.
Cardiologists, surgeons and patient spokesmen now share insights into new heart techniques via YouTube, LinkedIn and Instagram. These experts supported digital voices increase awareness of advanced facilities such as MRI compatibility, remote monitoring or main-free pacemakers, who guide the patient's interest in modern and premium solutions.
Digital health trends require other dentures integrated with Bluetooth or Wi-Fi features for smart pacemakers and other dentures synchronized with distance health platforms. These allow doctors to monitor the patient's condition in real time and adjust the settings without the need for frequent hospital visits-which is a rapidly valuable function in the post-pandemic care model.
Some manufacturers now offer other apps that are synchronized with heart units to allow both patients and doctors to track matrix, battery status and awake. The couple increases consumers' confidence in openness and authority technically advanced equipment.
Digital platforms provide interactive demos, webinars and training modules for health professionals for new heart units. These are not only accelerated, but also affect the purchase options for hospitals based on use and integration with existing systems.
Online tools and simulations now help patients understand the difference between prosthetic options (eg mechanically versus tissue heart valves), which allows for more informed, individual options for individual options.
Leading manufacturers invest in the adjustment of search engines (SEO) and payment advertising to improve visibility in findings related to heart prostheses. This is important as more online health professionals and decision -making equipment before buying or recommending digital appearance.
Through data analysis and AI, companies can offer personal ads and materials to hospital purchasing authorities and cardiologists on platforms such as Google and LinkedIn. This strategic goal helps to create brand preference and loyalty in a crowded market.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.96 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 16.7 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott., Artivion, Inc., Medtronic, Boston Scientific Corporation, Edwards Lifesciences Corporation., Siemens Healthcare Private Limited, LivaNova PLC (sorin group), Asahi Kasei Corporation., OSYPKA MEDICAL, Lepu Medical, MicroPort Scientific Corporation., Vitatron, Koninklijke Philips N.V., Progetti srl, and Shree Pacetronix Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients