Cardiac Implantable Electronic Device Market is estimated to be valued at USD 32.65 Bn in 2025 and is expected to reach USD 55.24 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.8% from 2025 to 2032.
Analysts’ Views on Global Cardiac Implantable Electronic Device Market:
Increasing geriatric population and rising incidence of cardiovascular diseases such as cardiac arrhythmia are expected to drive the global cardiac implantable electronic device market growth. For instance, in September 2022, Ceryx Medical, a company that develops novel implantable bioelectronics therapeutic device to target and treat heart failure, and Osypka Medical, a company that provides electrical cardiometry and temporary cardiac pacing medical devices, are developing a new type of heart pacemaker that will revolutionize how patients with heart failure and other cardiac diseases are treated.
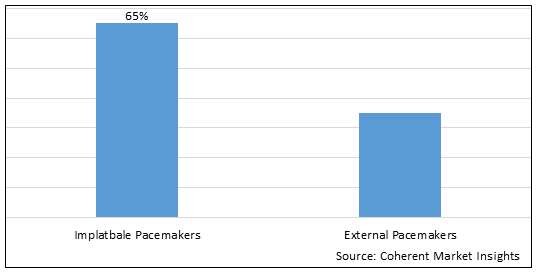
Figure 1. Global Cardiac Implantable Electronic Device Market Share (%), By Type, 2025
To learn more about this report, Download Free Sample
Global Cardiac Implantable Electronic Device Market– Drivers
Increasing Incidence of Cardiac Arrhythmia
Increasing prevalence of cardiac arrhythmia is expected to drive global cardiac implantable electronic device market growth over the forecast period. For instance, in April 2020, according to the Journal of Cardiovascular Diagnosis and Therapy, in the U.S, sudden cardiac death (SCD) causes 300,000 deaths annually. In the general population with cardiac issues, the reported incidence of SCD in Europe and North America ranges between 50 and 100 per 100,000. Ventricular tachyarrhythmia’s [ventricular tachycardia (VT) and ventricular fibrillation (VF)] are the most common mechanisms of SCD.
Increasing Launches of Cardiac Implantable Electronic Device
Increasing product launches to expand the portfolio is expected to drive the market growth over the forecast period. For instance, on June 12, 2023, Biotronik, a leader in implantable medical device technology, announced the first global implantation of its Biomonitor IV implantable cardiac monitor (ICM). Biomonitor IV is a cutting-edge ICM that combines artificial intelligence with Biotronik's Smart ECG technology to eliminate all false positive detections by 86% while keeping 98% of genuine events. It is also the only ICM that is capable of distinguishing between premature atrial contractions (PACs) and premature ventricular contractions (PVCs) to provide healthcare professionals with better tools for risk stratification and diagnosis.
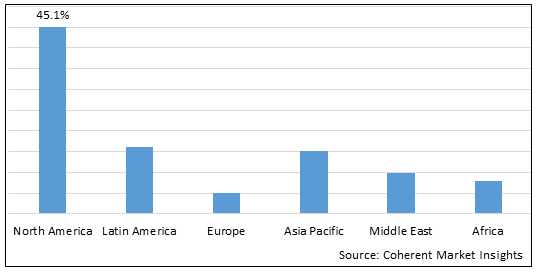
Figure 2. Global Cardiac Implantable Electronic Device Market Value (US$ Billion), By Region, 2025
To learn more about this report, Download Free Sample
Global Cardiac Implantable Electronic Device Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global cardiac implantable electronic device market over the forecast period, owing to increasing cardiovascular prevalence in the region. For instance, on May 15, 2025, according to the Centers for Disease Control and Prevention, in the U.S, about 805,000 people have heart attack, and one person dies every 33 seconds from cardiovascular disease annually.
Global Cardiac Implantable Electronic Device Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had negative impact on the global cardiac implantable electronic device market growth. Due to the COVID-19 outbreak, there had been decrease in the screening for cardiac diseases and cardiac rhythm monitoring. According to the study published by the Frontiers Public Health in November 2022, during the early phase of the COVID-19 pandemic, (cardiac implantable electronic device) CIED implantations decreased by 56.5% in northeastern Spain, 39.38% in Poland, 28% in the Veneto region of Italy, and 48% in northwestern Greece.
Cardiac Implantable Electronic Device Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 32.65 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.8% | 2032 Value Projection: | USD 55.24 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Biotronik, Boston Scientific Corporation or its affiliates, Pacetronix.com, Integer Holdings Corporation, Cook Group, Braile Biomédica, Abbott, LivaNova PLC, OSYPKA Medical, Galix Biomedical Instrumentation Inc., OSCOR Inc., LifeTech Scientific Corporation, MicroPort Scientific Corporation, and Ceryx Medical |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Cardiac Implantable Electronic Device Market- Segmentation
Global cardiac implantable electronic device market report is segmented into type, end user, and region.
Based on Type, the global cardiac implantable electronic device market is segmented into implantable pacemakers and external pacemakers. Out of which, implantable pacemakers segment is expected to dominate the global cardiac implantable electronic device market during the forecast period due to increasing prevalence of geriatric population and increasing cardiac arrhythmia.
Based on End User, the global cardiac implantable electronic device market is segmented into hospitals, specialty clinics, and ambulatory care settings. Hospitals segment is expected to dominate the market over the forecast period a due to increasing prevalence of cardiac diseases and geriatric population which lead to the admission of patients for the treatment purpose.
Based on Region, global cardiac implantable electronic device market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, the North America is expected to dominate the market over the forecast period owing to increasing launch of product by market key players
Among all segmentation, the type segment has the highest potential due to rising prevalence of geriatric population and cardiac arrhythmia over the forecast period. So, the key players are focused strategy such as product launch and partnership. For instance, on May 08, 2023, Biotronik, a multi-national cardiovascular biomedical research and technology company, received CE (Conformité Européenne) approval for the World’s First Pacemaker and (cardiac resynchronization therapy-pacemaker) CRT-P family approved for left bundle branch pacing.
Global Cardiac Implantable Electronic Device Market- Cross Sectional Analysis
Among type, the implantable pacemakers segment held a dominant position in North America region over the forecast period due to increasing number of cardiovascular diseases such as atrial fibrillation, and geriatric population. So, the market key players are focusing on the strategies such as acquisition and partnership for the market growth over the forecast period. For instance, on April 20, 2023, Aziyo Biologics, Inc., a company that develops and commercializes biologic products to improve compatibility between medical devices and the patients, announced that it had entered into a distribution agreement with LeMaitre Vascular, Inc., a provider of vascular devices, implants, and services. Under this agreement terms, Aziyo Biologics, Inc. will grant LeMaitre Vascular the exclusive U.S. distribution rights for the products within its cardiovascular segment.
Global Cardiac Implantable Electronic Device Market- Key Developments
In February 2022, Medtronic, a medical technology company that engages in the development, manufacturing, distribution, and sale of device-based medical therapies, announced that the Freezor and Freezor Xtra Cardiac Cryoablation Focal Catheters are the first and only ablation catheters approved by the U.S. Food and Drug Administration in August 2020 (FDA) to treat the growing prevalence of pediatric atrioventricular nodal reentrant tachycardia (AVNRT).
In January 2021, Boston Scientific Corporation or its affiliates, a pioneer in providing medical devices for less invasive surgical procedures, announced that it had entered into a definitive agreement to acquire Preventice Solutions, Inc., a privately-held company that offers a full portfolio of mobile cardiac health solutions and services ranging from ambulatory cardiac monitors including short and long-term Holter monitors, and cardiac event monitors and mobile cardiac telemetry. The transaction includes a US$ 925 million upfront cash payment and a potential US$ 300 million commercial milestone payment.
In January 2022, Medtronic, a medical technology company that engages in the development, manufacturing, distribution, and sale of device-based medical therapies, announced it had received approval from Japan's Ministry of Health, Labor and Welfare for the sale and reimbursement of the Micra AV Transcatheter Pacing System (TPS). This approval expands the number of patients in Japan, one of the largest markets in the world, who are eligible to receive the Micra TPS, the world's smallest pacemaker. The Micra AV is indicated for the treatment of patients with AV block, a condition in which the electrical signals between the chambers of the heart (the atria and the ventricle) are impaired.
In January 2020, Medtronic, a medical technology company that engages in the development, manufacturing, distribution, and sale of device-based medical therapies announced it had received CE (Conformité Européenne) Mark for its cobalt and crome portfolio of implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy-defibrillators (CRT-D). ICDs monitors heart rhythms and deliver therapy to correct heart rates that are too fast, and can lead to sudden cardiac arrest. CRT-Ds, a treatment option for some individuals with heart failure, sends small electrical impulses to the lower chambers of the heart to help them beat in more synchronized patterns and reduce patient symptoms.
Global Cardiac Implantable Electronic Device Market- Key Trends
Innovation in Implantable Electronic Devices
Innovation in implantable electronic devices is expected to drive the global implantable electronic device market growth over the forecast period. For instance, in June 2021, Medtronic, a medical technology company thatengages in the development, manufacturing, distribution, and sale of device-based medical therapies, announced the initiation of the DEFINE AFib study, the company's first app-based research study. By suing data collected from the LINQ family of insertable cardiac monitors (ICMs), the purpose of the study is to explore unresolved issues about the burden of atrial fibrillation (AF) and its effects on patient outcomes, quality of life, and healthcare use.
Global Cardiac Implantable Electronic Device Market: Restraints
Product Recall by the Key Players and Regulatory Authority
Product recall by the key market players and regulatory authority is expected to restrict the global cardiac implantable electronic device market growth over the forecast period. For instance, in February 2021, Medtronic, a medical technology company that engages in the development, manufacturing, distribution, and sale of device-based medical therapies, issued a recall notice for Evera, Viva, Brava, Claria, Amplia, Compia, and Visia Implantable Cardioverter Defibrillators (ICDs), and Cardiac Resynchronization Therapy (CRT-Ds) due to risk of shortened battery life. The company had issue a notice about the compensation for the buyers due to the urgent recall of the medical devices. So, to avoid this, a large battery life devices to be employed as a pacemakers.
Global Cardiac Implantable Electronic Device Market- Key Players
Major players operating in the global cardiac implantable electronic device market include Medtronic, Biotronik, Boston Scientific Corporation or its affiliates, Pacetronix.com, Integer Holdings Corporation, Cook Group, Braile Biomédica, Abbott, LivaNova PLC, OSYPKA Medical, Galix Biomedical Instrumentation Inc., OSCOR Inc., LifeTech Scientific Corporation, MicroPort Scientific Corporation, and Ceryx Medical.
*Definition: Cardiac implantable electronic device (CIED) replacement is a surgery to replace a device that helps to control heart rhythm. The procedure can replace an implantable cardioverter defibrillator (ICD) or a pacemaker. An implantable cardiac defibrillator (ICD) is an electronic device that is placed inside the body. An ICD constantly keeps track of heart rhythm and sends a small shock to the heart muscle if the rhythm becomes abnormal (arrhythmia). Cardiovascular implantable electronic device (CIED) consists of pacemakers for Brady arrhythmia treatment, implantable cardioverter defibrillators (ICDs) for tachyarrhythmia management, and cardiac resynchronization therapy (CRT) devices for systolic dysfunction with conduction delays.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients