Cancer Vaccines Market Size and Forecast – 2025 – 2032
The Global Cancer Vaccines Market size is estimated to be valued at USD 6.8 billion in 2025 and is expected to reach USD 12.9 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.5% from 2025 to 2032.
Global Cancer Vaccines Market Overview
Cancer vaccines are a form of immunotherapy designed to prevent or treat cancer by stimulating the body’s immune system to recognize and attack cancer cells. Unlike traditional vaccines that prevent infections, therapeutic cancer vaccines target existing tumors by introducing antigens specific to cancer cells. Some, like the HPV and Hepatitis B vaccines, prevent virus-related cancers. Others, such as Provenge for prostate cancer, are used after diagnosis. Research is advancing rapidly, with personalized vaccines being developed based on a patient’s unique tumor profile. Cancer vaccines offer a promising, targeted approach to treatment, aiming to boost immune response with fewer side effects.
Key Takeaways
Therapeutic vaccines lead the market share within vaccine type segments, capitalizing on their specificity and ease of manufacturing, making them a dominant force in cancer vaccines size and revenues.
Lung cancer dominates the cancer type segment owing to its high incidence, with notable vaccine development focused on this area reflecting significant market revenue opportunities.
Hospitals represent the largest end-user segment, driven by expanding oncology departments and the adoption of innovative immunotherapies.
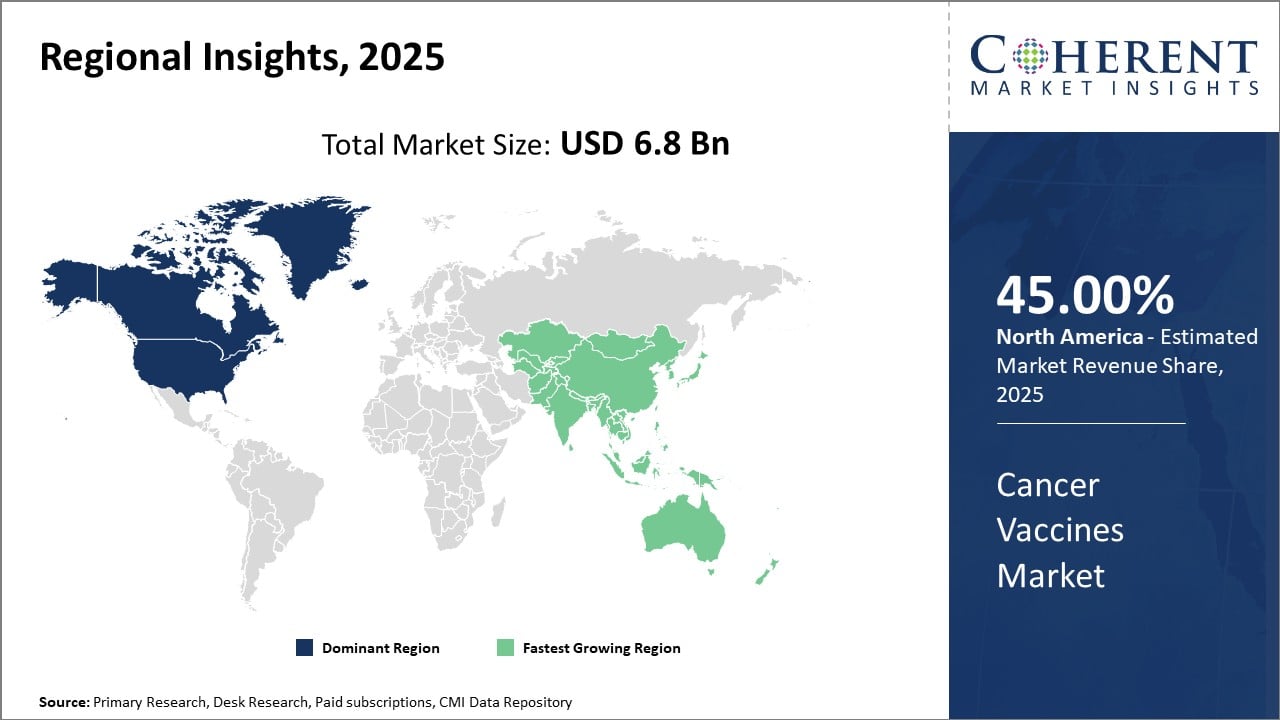
North America remains the dominant regional market, accounting for approximately 45% of the market share, underpinned by robust infrastructure and R&D investment.
Asia Pacific emerges as the fastest-growing region with a CAGR exceeding 11%, propelled by increasing government initiatives and rising incidence rates in populous countries like China and India.
Cancer Vaccines Market Segmentation Analysis
To learn more about this report, Download Free Sample
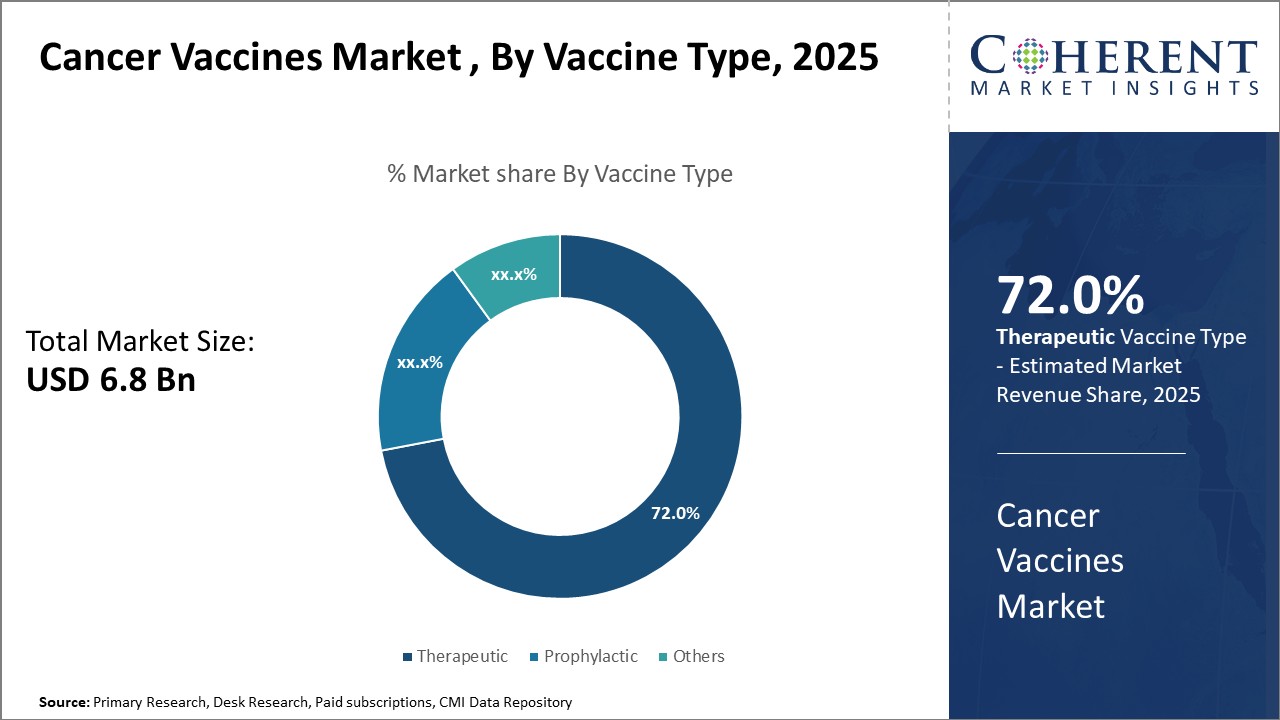
Cancer Vaccines Market Insights, By Vaccine Type
In terms of vaccine type, Therapeutic vaccines dominate the market share with 72%. Peptide vaccines are favored due to their relatively straightforward manufacturing processes and ability to target well-defined tumor-associated antigens, driving extensive clinical adoption. This segment benefits significantly from ongoing advancements in synthetic peptide technology, resulting in improved immunogenicity and patient-specific customization.
Cancer Vaccines Market Insights, By Cancer Type
In the cancer type segment, Lung cancer dominates the market share at 28%. This is attributed to the high global incidence rate and the urgent need for efficacious therapies. Vaccine development targeting lung cancer has benefited from identifying specific tumor antigens like MUC1 and EGFR mutations, driving pipeline focus. Melanoma stands out as the fastest-growing segment due to favorable immune-related response rates and successful vaccination campaigns in clinical settings.
Cancer Vaccines Market Insights, By End-User
The Hospital segment is dominating the market share at 40%. This dominance is explained by the presence of advanced oncology wards equipped to administer complex immunotherapies and the growing standardization of vaccines in cancer treatment protocols. Hospitals also benefit from larger budgets and infrastructure, facilitating early adoption of innovative vaccine therapies. The fastest-growing subsegment is cancer research institutes, which play a critical role in clinical trial support and translational research enhancing vaccine efficacy and specificity.
Cancer Vaccines Market Trends
The Cancer Vaccines market trend is increasingly dominated by personalized therapeutic approaches tailored to tumor-specific antigens.
This is supported by increased R&D spending on neoantigen vaccines, complementing conventional peptide vaccines, backed by successful phase II and III clinical trials in 2024.
AI integration in predictive immunology platforms is enhancing the precision and speed of vaccine development, reducing time to market by nearly 30% while increasing efficacy.
Another pivotal trend is the move toward combination therapies pairing vaccines with immune checkpoint inhibitors or targeted therapies, which have demonstrated increased overall survival rates and patient reactivity in multi-center trials conducted across the U.S. and Europe.
Cancer Vaccines Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Cancer Vaccines Market Analysis and Trends
In North America, the dominance in the Cancer Vaccines market is driven by significant investments in oncology research, superior healthcare infrastructure, and a strong presence of market players such as ImmunoGenix and BioVaxTech. The region holds roughly 45% of the global market share, supported by early adoption of novel therapies and supportive regulatory frameworks, fostering rapid commercialization.
Asia Pacific Cancer Vaccines Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 11%. This surge is propelled by rising cancer incidence rates, expanding healthcare infrastructure, and increasing government support in countries like China and India. Notable contributions from companies such as NeoVax Bio and CelluVax Inc. focus on localized vaccine development, enhancing the region's potential.
Cancer Vaccines Market Outlook for Key Countries
USA Cancer Vaccines Market Analysis and Trends
The USA’s cancer vaccines stand as the largest globally, driven by early innovation and large-scale clinical trials. In 2024, more than USD 3.2 billion was invested in cancer vaccine R&D, accounting for almost 47% of overall market revenue. Major players like ImmunoGenix have leveraged accelerated FDA approvals for their dendritic cell vaccines, shortening commercialization cycles by 20%. Additionally, collaboration between biopharma companies and federal institutions has expedited breakthroughs in lung and melanoma vaccine candidates, reinforcing the country’s leadership in the market.
China Cancer Vaccines Market Analysis and Trends
China's cancer vaccines are rapidly expanding, with government initiatives boosting biopharma investments by 15% year-over-year in 2024 and a growing aging population increasing antigen-specific therapy demand. Domestic companies such as NeoVax Bio focus on peptide vaccine platforms customized for regional cancer profiles, contributing to 30% of the Asia Pacific market revenue. China's regulatory reforms, facilitating faster clinical trial approvals, have accelerated product launches, positioning the country as the second-largest market ahead of Europe.
Analyst Opinion
● The surge in clinical trials focusing on dendritic cell vaccines and peptide-based vaccines underscores the growing supply-side production capacity enhancements within the cancer vaccines market. In 2024 alone, over 150 active trials worldwide reported increased enrollment rates, suggesting heightened clinical confidence in vaccine candidates targeting melanoma and prostate cancer.
● Demand-side indicators highlight a remarkable rise in adoption across emerging economies, driven by greater healthcare expenditure and increasing incidences of lung and colorectal cancers. For instance, Latin American countries reported a 12% increase in cancer vaccine imports in 2024 as hospitals sought advanced immunotherapies.
● Micro-indicators such as pricing strategies have adjusted to the competitive landscape with tiered pricing allowing penetration into cost-sensitive regions, boosting revenue streams by 8% in Asia Pacific markets between 2023 and 2024.
● The nano-scale innovation in nanoparticle-based vaccine delivery platforms contributed to enhanced antigen targeting and immune response. Recent studies published in 2025 noted a 15% increase in vaccine efficacy using nanoformulated adjuvants, particularly for personalized cancer vaccines targeting neoantigens.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 6.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.5% | 2032 Value Projection: | USD 12.9 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | BioVaxTech; ImmunoGenix; ProMedica Therapeutics; NeoVax Bio; CelluVax Inc.; OncoVax Solutions; MedGene Biologics; ViroThera LLC; Genetix Pharmaceuticals; ImmuneFocus; NanoVax Corp.; OncoShot Biotech. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Cancer Vaccines Market Growth Factors
Rapid developments in immunotherapy technologies, especially in peptide and dendritic cell vaccines, continue to drive the market growth. Increasing funding for cancer research globally, notably from governmental bodies such as the National Cancer Institute (NCI) in the U.S., which allocated over USD 6 billion in 2024 for vaccine-related projects, accelerates innovation and expansion.
Furthermore, rising cancer prevalence worldwide, with lung and colorectal cancers leading in incidence as reported by the WHO in their 2024 statistics, underscores the urgency and business growth opportunities for cancer vaccines. Regulatory progression easing expedited approvals for breakthrough therapies also supports swift market penetration and revenue growth, evidenced by multiple fast-tracked vaccine approvals in Europe and North America during 2024-2025.
Cancer Vaccines Market Development
In January 2023, India’s Serum Institute of India (SII) launched CERVAVAC, the country’s first indigenously developed quadrivalent human papillomavirus (qHPV) vaccine aimed at the prevention of cervical cancer. The vaccine was developed through a collaboration between SII, the Department of Biotechnology (DBT), and the Bill & Melinda Gates Foundation. CERVAVAC is designed to protect against HPV types 6, 11, 16, and 18, which are responsible for the majority of cervical cancer cases worldwide.
In March 2025, researchers at the Icahn School of Medicine at Mount Sinai announced promising results from a phase 1 trial of their personalized multi-peptide neoantigen vaccine (PGV001). The vaccine, designed using patient-specific tumor mutations, was shown to generate strong immune responses in patients who had a high risk of relapse after standard treatments.
Key Players
Leading Companies of the Market
BioVaxTech
ImmunoGenix
ProMedica Therapeutics
NeoVax Bio
CelluVax Inc.
MedGene Biologics
Genetix Pharmaceuticals
ImmuneFocus
NanoVax Corp.
OncoShot Biotech
Several companies have adopted competitive strategies such as mergers and acquisition to accelerate innovation; for example, BioVaxTech’s acquisition of OncoShot Biotech in early 2024 expanded its technological capabilities in nano-delivery systems, resulting in a 20% increase in its product pipeline velocity within six months. Meanwhile, ImmunoGenix’s strategic collaboration with leading research institutes in North America fostered faster clinical trials, reducing time to market by approximately 18%.
Cancer Vaccines Market Future Outlook
The cancer vaccines are expected to become an integral part of personalized oncology, driven by breakthroughs in genomics, biomarker discovery, and immune engineering. Therapeutic vaccines tailored to tumor antigens and patient-specific immune profiles will complement existing therapies like checkpoint inhibitors and CAR-T cells, enhancing treatment outcomes. The adoption of mRNA platforms is anticipated to revolutionize vaccine pipelines by reducing development timelines and improving scalability. Expanding collaborations between pharmaceutical companies, biotech innovators, and research institutions will play a crucial role in advancing commercialization. With increasing regulatory support, growing investment, and a rising demand for more targeted cancer therapies, the cancer vaccines segment is positioned for strong long-term growth as part of the wider immuno-oncology ecosystem.
Cancer Vaccines Market Evolution
The cancer vaccines landscape has evolved steadily over the past decade, shaped by the rising burden of cancer worldwide and the shift toward immunotherapy as a frontline treatment. Preventive vaccines, such as those for HPV and hepatitis B, established the foundation by reducing infection-related cancers, while therapeutic vaccines gradually gained traction through advances in biotechnology and oncology research. Early challenges around efficacy and immune response limited widespread adoption, but the integration of adjuvants, improved delivery platforms, and the success of checkpoint inhibitors helped validate the potential of cancer vaccines.
Sources
Primary Research interviews:
Oncology Researchers
Vaccine Development Scientists
Immunologists
Clinical Trial Investigators
Databases:
National Cancer Institute (NCI)
World Health Organization (WHO) Database
Centers for Disease Control and Prevention (CDC) Database
Magazines:
Nature Reviews Cancer
Science News
PharmaTimes
BioWorld
Medical News Today
Health Affairs
Journals:
Journal of Hematology & Oncology
Molecular Aspects of Medicine
ACS Biomaterials Science & Engineering
Cancer Immunology Research
Newspapers:
The Guardian (Health)
Reuters Health
The Washington Post (Health & Science)
Associations:
Cancer Research Institute (CRI)
American Association for Cancer Research (AACR)
Cancer Vaccine Institute (CVI)
World Cancer Research Fund
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients