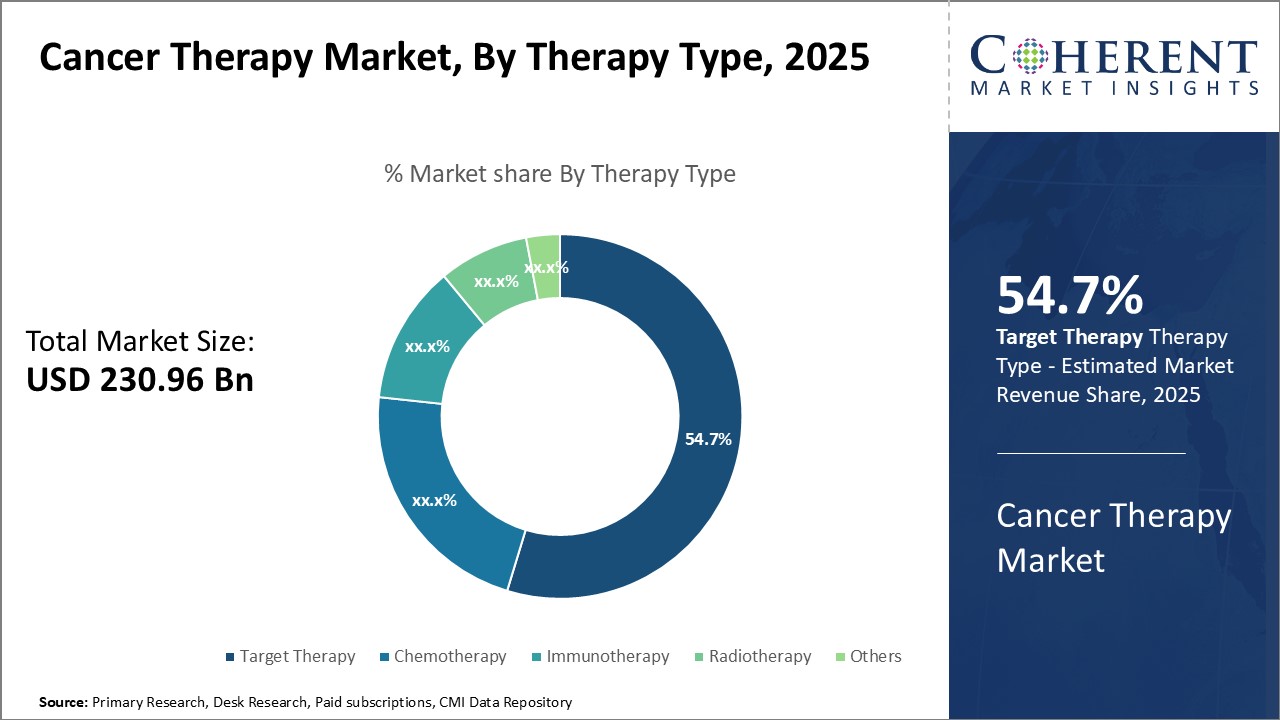
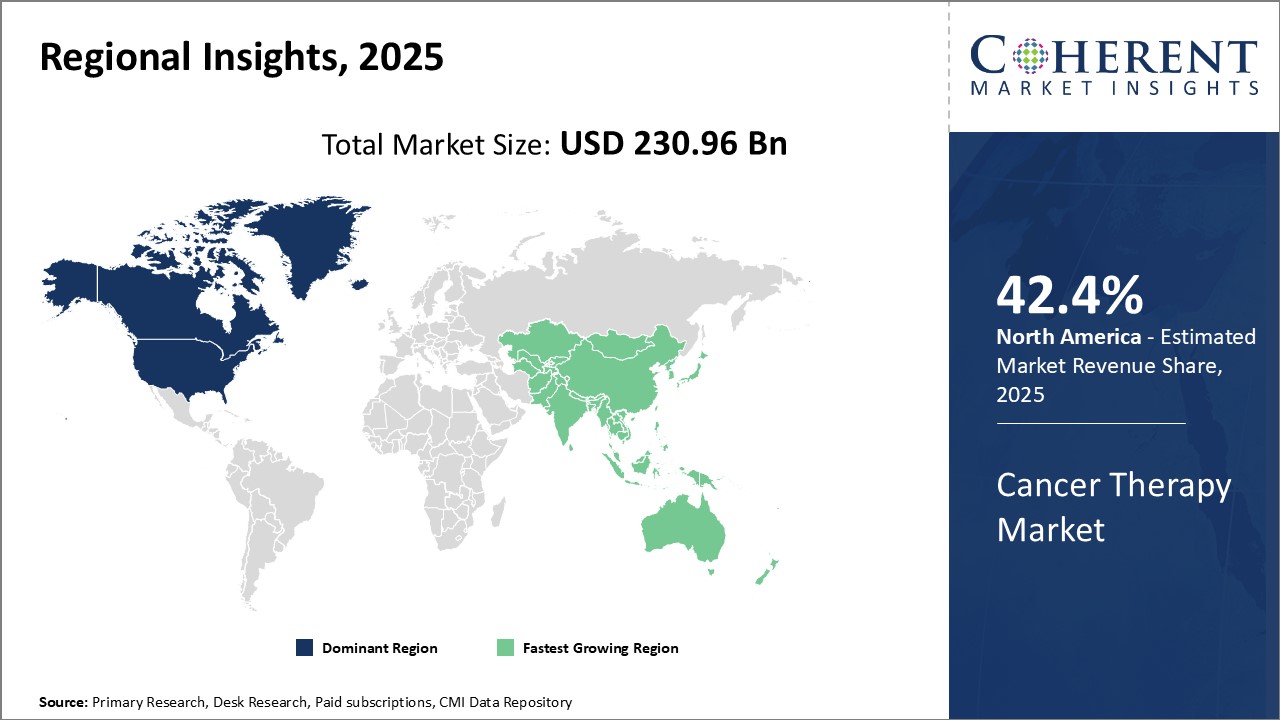
The cancer therapy market is estimated to be valued at USD 230.96 Bn in 2025 and is expected to reach USD 530.37 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
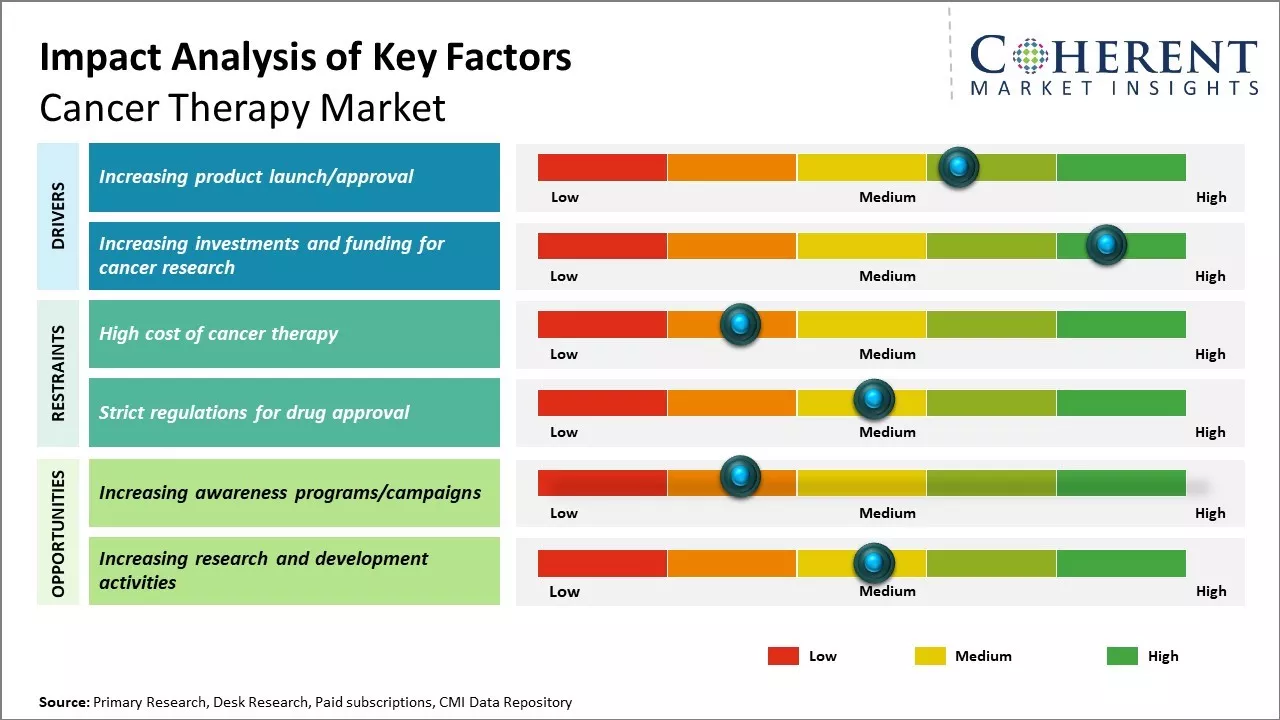
The cancer therapy market is expected to witness high growth during the forecast period. Increasing product launches/approvals and investments and funding for cancer research are major drivers. Additionally, the increasing prevalence of cancer worldwide is expected to propel the demand for novel targeted drugs for cancer treatment. Further advancement in cancer diagnostic techniques resulting in early detection will also positively impact the market growth. High cost of treatment for various cancers still remains a restraint especially in developing nations. The market also faces challenges due to stringent regulatory approvals for new therapies. Among all regions, Asia Pacific is likely to witness the fastest growth rate owing to a rise in healthcare expenditure and improving access to modern therapies in emerging economies like China and India. North America will continue to dominate the market supported by well-established healthcare infrastructure and increasing spending on specialty clinics.
Increasing product launch/approval
The increasing number of product approvals and launches for cancer treatment over the past couple of years has provided a significant boost to the cancer therapy market. For instance, in January 2023, the European Union (EU) approved Enhertu (trastuzumab deruxtecan) as monotherapy for the treatment of adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer. Enhertu (trastuzumab deruxtecan) is being jointly developed and commercialized by AstraZeneca, a pharmaceutical company, and Daiichi Sankyo, Limited, a global biopharmaceutical company. Enhertu is a specifically engineered HER2-directed antibody drug conjugate (ADC). Moreover, in December, 2022, Mirati Therapeutics, Inc., a biotechnology company, announced that it had received accelerated approval from the U.S. Food and Drug Administration for adagrasib, a RAS GTPase family inhibitor, for adult patients with KRAS G12C mutated locally advanced or metastatic non-small cell lung cancer (NSCLC).
Get actionable strategies to beat competition: Download Free Sample
Increasing investments and funding for cancer researchMany governments allocate substantial funds to cancer research through grants to universities, medical institutions, and research organizations. National health institutes and cancer research centers are major recipients where innovations in diagnostics, treatments, and prevention methods are frequently developed, there has been increase in the funding provided by government organizations and private investors for conducting research and development activities. For instance, in March 2023, Volastra Therapeutics, a clinical-stage cancer biotechnology company, secured USD 60 million in series A funding. The company intends to use the funds to advance clinical pipeline of both sovilnesib and VLS-1488 in 2023. Sovilnesib is currently in Phase 1 for the treatment of platinum-resistant high-grade serous ovarian cancer, triple-negative breast cancer, and other solid tumors with TP53 mutations. It has also received the U.S. Food and Drug Administration’s fast-track designation in platinum-resistant high grade serous ovarian cancer, underscoring the high unmet need in this population.

To learn more about this report, Download Free Sample
Market Challenges – Strict regulations for drug approvalThe U.S. Food and Drug Administration (FDA) protects the quality of medicinal products by closely monitoring drug manufacturers' adherence to U.S. FDA's current good manufacturing practices (CGMP) requirements. The CGMP rules for medications provide minimum standards for the procedures, facilities, and controls used in producing, processing, and packaging a drug product. The rules ensure that a product is safe to use and has the ingredients that are required. Transformation in the drug approval regulations is expected to hinder the market growth. For instance, in November 2021, a high-powered committee was formed in India to suggest improvements to the Indian drug regulatory system that entailed reorganizing the Drug Controller General of India's Central Drugs Standard Control Organization. Therefore, strict regulations from the national and international governments on drug safety and efficacy assessment to protect health of the patients are expected to hinder the market growth.
Market Opportunities – Increasing awareness programs/campaigns
The increasing adoption of various initiatives such as awareness programs, campaigns, and others for the treatment of cancer by key market players, organizations, and governments of respective countries is expected to foster the market growth over the forecast period. For instance, in November 2022, the government of Canada, initiated Lung Cancer Awareness Month, to raise awareness about lung cancer and the actions to help prevent it and reduce the stigma associated with this disease. Moreover, in October 2022, the government of the U.S. conducted lung cancer awareness month to create awareness on the prevention and treatment of patients suffering from lung cancer.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Therapy Type: Continuous Development of New and Improved Targeted DrugsThe therapy type segment includes chemotherapy, target therapy, immunotherapy, radiotherapy, and others. The target therapy segment is estimated to hold 54.7% share of the market in 2025. Target therapy has contributed significantly to the global cancer therapy market by delivering more precise and effective treatments. This highly targeted approach works by blocking the growth and spread of cancer cells while limiting harm to healthy cells. Target therapies focus on specific molecular changes or genetic defects within a tumor that help cancer cells grow and survive. Key market players are engaged in developing new treatment solutions for various cancer types, in order to get product approvals from regulatory authorities, which is expected to boost the segment growth over the forecast period. For instance, in September 2022, Eli Lilly and Company, a pharmaceutical company, announced the U.S. Food and Drug Administration (FDA) had granted approval to Retevmo (selpercatinib) for adult patients with locally advanced or metastatic solid tumors with a rearranged during transfection (RET) gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options.
Insights, By Cancer Type: Breast Cancer Screening Drives Incidences and Treatment
The cancer type segment includes Breast Cancer, Lung Cancer, Colorectal Cancer, Prostate Cancer, Blood Cancer, Liver Cancer, Thyroid Cancer, Head and Neck Cancer, Skin Cancer, and Others. The breast cancer subsegment is expected to have 15.3% of the market share in 2025. Of the major cancer types, breast cancer accounts for the highest percentage of the global cancer therapy market due to its prevalence and established screening protocols. Regular breast examinations and mammography have proven highly effective at detecting tumors in early, most treatable stages. As a result, breast cancer incident rates have steadily increased over time rather than decreased mortality. Widespread public health campaigns in many Western nations have normalized annual checkups from age 40 onward. Compliance with these programs helps ensure fast diagnosis and timely intervention, which boosts the need for both surgical and drug-based therapies. Even in developing regions, education efforts have improved breast health awareness and access to screening services.
Need a Different Region or Segment? Download Free Sample
North America remains the dominant region in the global cancer therapy market and is anticipated to hold 42.4% of the market share in 2025. Increasing product approvals by regulatory authorities are expected to drive the North America cancer therapy market growth over the forecast period. For instance, on February 9, 2023, GSK plc., a biopharmaceutical company, announced that it had received approval from the U.S. Food and Drug Administration for Jemperli (dostarlimab-gxly) for the treatment of adult patients with mismatch repair-deficient (dMMR) recurrent or advanced endometrial cancer.
Overall, the Asia Pacific region offers pharmaceutical companies attractive opportunities due to its demographic and economic trends. With a more favorable regulatory environment emerging and urbanization amplifying healthcare demands, the market potential continues expanding at a rapid pace. If current momentum sustains, Asia Pacific may rival and even surpass North America as the top market for cancer therapies in the long run.
Cancer Therapy Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 230.96 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.6% | 2032 Value Projection: | USD 530.37 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
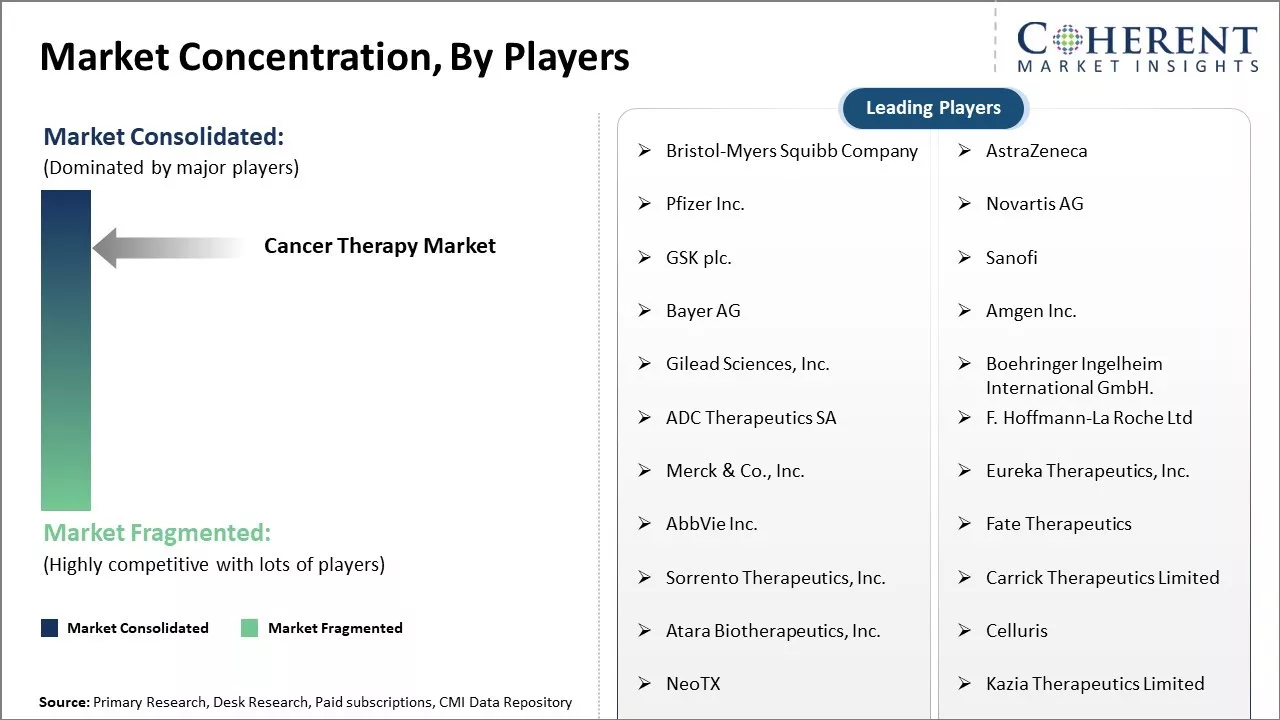
| Companies covered: |
Bristol-Myers Squibb Company, AstraZeneca, Pfizer Inc., Novartis AG, GSK plc., Sanofi, Bayer AG, Amgen Inc., Gilead Sciences, Inc., Boehringer Ingelheim International GmbH., ADC Therapeutics SA, F. Hoffmann-La Roche Ltd, Merck & Co., Inc., Eureka Therapeutics, Inc., AbbVie Inc., Fate Therapeutics, Sorrento Therapeutics, Inc., Carrick Therapeutics Limited, Atara Biotherapeutics, Inc., Celluris, NeoTX, Kazia Therapeutics Limited |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Cancer therapy refers to the use of various treatments such as surgery, radiation, medications, and other therapies to cure cancer, shrink tumors, or stop cancer progression. These treatments include chemotherapy, radiation therapy, immunotherapy, hormone therapy, targeted drug therapy, bone marrow transplants, cryoablation, radiofrequency ablation, and participation in clinical trials.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients