Cancer Biologics Market Size and Forecast – 2026 – 2033
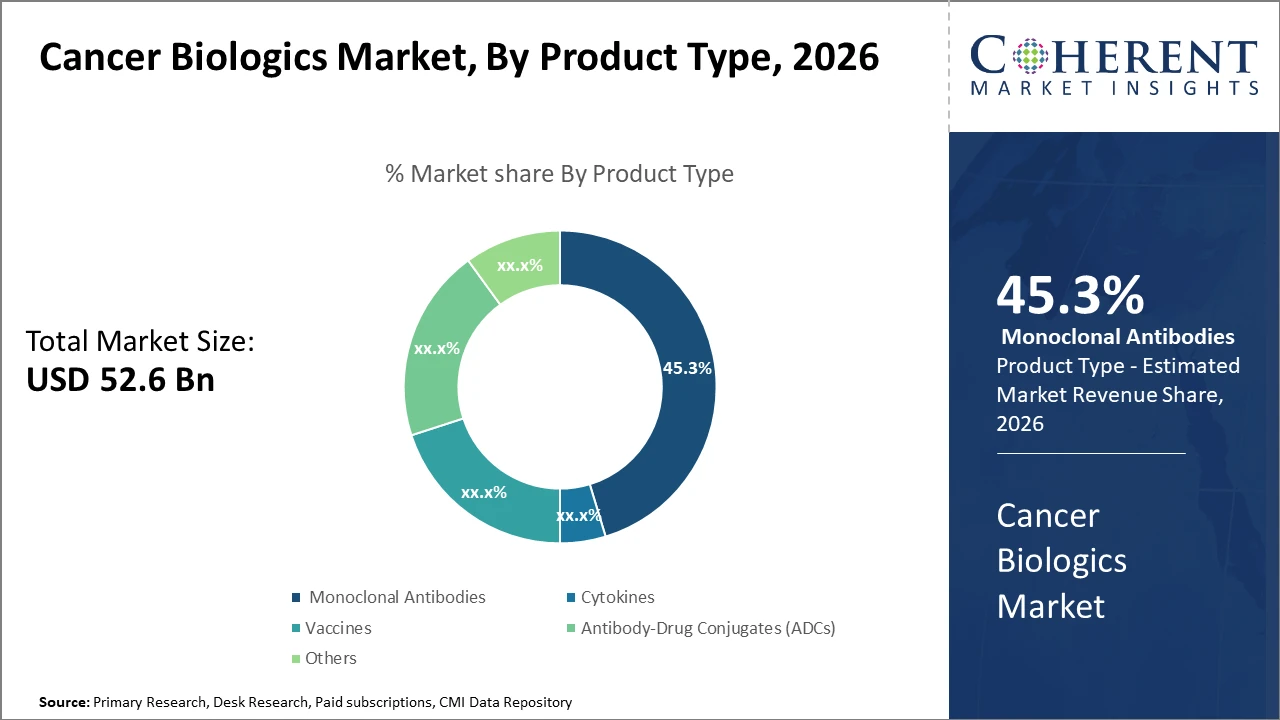
The Global Cancer Biologics Market size is estimated to be valued at USD 52.6 billion in 2026 and is expected to reach USD 96.3 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 8.6% from 2026 to 2033.
Global Cancer Biologics Market Overview
Cancer biologics are therapeutic products derived from living organisms or biological processes and are used to treat various types of cancer. These include monoclonal antibodies, therapeutic vaccines, cytokines, and cell-based therapies. Cancer biologics work by targeting specific cancer cells, enhancing immune response, or inhibiting tumor growth pathways. These products are administered through injectable or infusion-based delivery systems and are widely used in oncology treatment protocols.
Key Takeaways
Product segments such as Monoclonal Antibodies dominate the market with 45.3% industry share, driven by their established clinical efficacy and multiple approved indications.
Hematologic Malignancies represent the largest therapeutic application segment, constituting nearly 40% of the market due to persistent unmet clinical needs and targeted therapeutic benefits.
Hospitals remain the critical end-user segment with above 50% revenue capture, fueled by increasing oncology patient volumes and specialized care infrastructure.
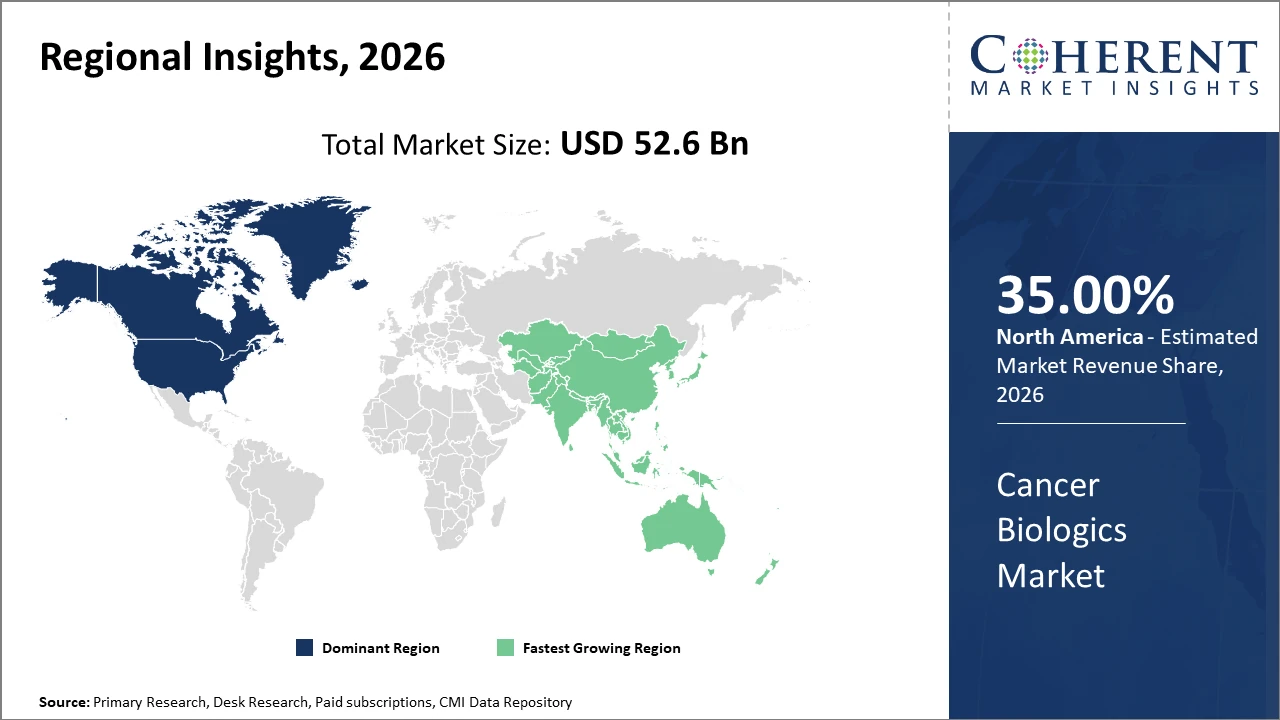
Regionally, North America holds the commanding market share at 35% globally, attributable to a robust biotech ecosystem, progressive regulatory framework, and significant investment in oncology research.
Asia Pacific is the fastest-growing region, exhibiting an above 9% CAGR supported by expanding healthcare infrastructure, increased awareness, and growing adoption of biologics.
Europe maintains steady growth, bolstered by an established pharmaceutical infrastructure and favorable reimbursement policies.
Cancer Biologics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Cancer Biologics Market Insights, By Product Type
Monoclonal Antibodies dominate the market share. Monoclonal antibodies maintain dominance due to their broad spectrum of clinical approvals and proven efficacy across multiple cancer indications, accounting for nearly half of the total market revenue. Antibody-Drug Conjugates are the fastest-growing subsegment given their novel mechanism combining targeted therapy with cytotoxic agents, driving high clinical trial activity and increasing adoption. Cytokines hold a stable position, appreciated for their role in modulating immune response. Vaccines are witnessing increased research investment especially in personalized cancer vaccines, promising future market growth.
Cancer Biologics Market Insights, By Application
Hematologic Malignancies dominate market share due to persistent unmet clinical needs and high prevalence; it accounts for the largest industry share as these biologics provide targeted efficacy in leukemia and lymphoma. The fastest-growing subsegment is Immune Checkpoint Inhibitors, which are revolutionizing solid tumor treatments by enhancing immune system targeting of cancer cells, gaining traction through expanded indications and approvals. Solid tumors remain a significant segment encompassing biologics across lung, breast, and colorectal cancers. Personalized cancer vaccines are an emerging focus, leveraging neoantigen-specific therapy to maximize immune responses.
Cancer Biologics Market Insights, By End-User
Hospitals dominate the market share since they serve as the primary settings for cancer biologics administration, accounting for over half of the industry revenue; extensive oncology units and clinical infrastructure fuel this segment. The fastest-growing subsegment is Specialty Clinics, which benefits from increasing specialization in cancer treatment and improved patient access to novel biologics outside hospital settings. Research Institutes remain significant for clinical trials and early-stage biologic development.
Cancer Biologics Market Trends
The evolving market is profoundly influenced by three main trends.
First is the surge in personalized medicine, powered by advances in genomic sequencing, allowing therapies to be tailored based on individual tumor profiles.
For example, CAR-T therapies secured multiple FDA approvals for indications in 2026, demonstrating the shift towards cellular immunotherapies.
Second, biosimilars have accelerated market transformation by providing more affordable alternatives to high-cost biologics, with biosimilar uptake increasing by 18% in North America during 2026 alone.
Third, digital health applications, including remote patient monitoring and AI-driven diagnostics, are enhancing therapeutic outcomes and market penetration.
Cancer Biologics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Cancer Biologics Market Analysis and Trends
In North America, the dominance in the Cancer Biologics market stems from strong R&D infrastructure, high healthcare expenditure, and accelerated regulatory approvals. The U.S. leads with nearly 40% market share, supported by biotech giants like Roche and Amgen headquartered here. Government initiatives to enhance cancer care and payer reforms have also streamlined patient access to biologics.
Asia Pacific Cancer Biologics Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 9%, fueled by burgeoning healthcare investments, rising cancer prevalence, and expanding manufacturing capabilities. Countries like China and India benefit from increasing clinical trial activities and biosimilar adoption. Notable regional companies like Biocon have significantly contributed to this expansion by developing cost-effective biologics.
Cancer Biologics Market Outlook for Key Countries
USA Cancer Biologics Market Analysis and Trends
The USA's Cancer Biologics market remains the largest globally, driven by well-established pharmaceutical companies, advanced healthcare infrastructure, and favorable government policies promoting innovation. In 2026, the U.S. witnessed a 12% increase in biologics approvals, including immune checkpoint inhibitors and ADCs, propelling market revenue upwards. Payers' increasing reimbursement for advanced therapies and high public and private R&D investment contribute to the country’s leading position.
China Cancer Biologics Market Analysis and Trends
China’s market has emerged as a critical growth engine, with national healthcare reforms enhancing biologics accessibility. The government’s support for biotech innovation and local manufacturing of biosimilars led to a 15% increase in cancer biologics market revenue in 2026. Partnerships between global players and local firms have accelerated product launches, making China one of the pivotal countries in shaping future market dynamics.
Analyst Opinion
Rising Production Capacity and Technology Adoption: Manufacturing efficiencies have ramped up markedly, with global biologics production capacities expanding by over 15% in 2026 alone. This expansion enables better supply-side responsiveness to escalating global demand, especially for monoclonal antibodies, which accounted for over 45% of the industry's market share in 2026.
Diversified Application Across Oncology Segments: Demand-side analysis shows the broad adoption of cancer biologics in hematologic malignancies and solid tumors alike, with emerging uses in immunotherapy yielding increased import volumes into key markets such as the U.S. and Europe. In 2026, import values of innovative cancer biologics products increased by 12%, showing growing treatment penetration.
Strategic Pricing Dynamics: Competitive pricing strategies among market players have influenced adoption rates, with biosimilars contributing to making therapies more accessible. Pricing adjustments have also aligned with reimbursement policies in key regions, driving a 7% increase in patient access year over year.
Expanding Use Cases in Adjacent Therapies: Nano-formulated biologics and combination therapies have started gaining traction, evidenced by clinical trial registrations increasing by 20% in 2026 compared to prior years. These micro-innovations significantly enhance therapeutic efficacy and patient compliance, impacting the market revenue positively.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2026 | Market Size in 2026: | USD 1.2 billion |
| Historical Data for: | 2021 To 2025 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.1% | 2033 Value Projection: | USD 1.73 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Eli Lilly and Company, AbbVie, Gilead Sciences, Sanofi, Takeda Pharmaceutical, Celgene (A Bristol Myers Squibb Company), Bayer AG, CSL Limited, Sorrento Therapeutics, Seattle Genetics. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Cancer Biologics Market Growth Factors
The market growth is primarily driven by the rising global cancer burden demanding improved biologic treatment solutions, with cancer incidence rates increasing by 19% globally since 2021. Advances in molecular biology and genetic engineering have propelled R&D efficiency, enabling the rapid introduction of targeted therapies and personalized biologics. Regulatory incentives across North America, Europe, and parts of Asia have accelerated clinical trials and approvals, optimizing time-to-market for novel biologics. Additionally, growing governmental and private funding directed towards biologics research has enhanced market scope and business growth opportunities, evident from a 14% increase in R&D expenditures in 2026.
Cancer Biologics Market Development
In January 2025, Roche received regulatory approval for Polivy (polatuzumab vedotin) for the treatment of lymphoma, including diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). The approval reinforced Polivy’s role as a targeted antibody–drug conjugate, offering improved therapeutic outcomes for patients with relapsed or refractory disease by delivering cytotoxic therapy directly to malignant B cells.
In April 2025, Amgen secured European approval for Blincyto (blinatumomab) in patients with Philadelphia chromosome–positive (Ph+) B-cell acute lymphoblastic leukemia (ALL). This milestone expanded Blincyto’s clinical utility as a bispecific T-cell engager (BiTE), enabling more precise immune-mediated targeting of leukemia cells and advancing treatment options for high-risk hematologic malignancies.
Key Players
Leading Companies of the Market
Eli Lilly and Company
AbbVie
Gilead Sciences
Sanofi
Takeda Pharmaceutical
Celgene (A Bristol Myers Squibb Company)
Bayer AG
CSL Limited
Sorrento Therapeutics
Seattle Genetics
Competitive strategies include Roche’s adaptive licensing approach that expedited market entry for its monoclonal antibody therapies, resulting in a 10% increase in market revenue within 18 months post-launch. Similarly, Amgen’s strategic alliance with Asian biotech firms expanded its footprint in emerging markets, resulting in a 15% sales growth in 2026. Another notable example is Bristol-Myers Squibb’s investment in novel ADC platform,s which increased its cancer biologics portfolio’s clinical pipeline by 25% over the last two years.
Cancer Biologics Market Future Outlook
The cancer biologics market is poised for continued expansion as precision medicine, gene-editing technologies, and personalized immunotherapies mature. Next-generation biologics will focus on bispecific antibodies, multi-targeted constructs, and combination regimens that harness the immune system more effectively with fewer side effects. Advances in biomarkers and companion diagnostics will enable more tailored treatment selection, improving patient outcomes. Novel delivery systems and engineered protein formats will enhance tissue penetration and reduce systemic toxicity. As global healthcare systems confront rising cancer incidence and demand for affordable biologics increases, strategic partnerships and biosimilar deployment will shape competitive dynamics while fostering broader access to life-saving therapies.
Cancer Biologics Market Historical Analysis
The market has transformed oncology treatments over the past several decades, beginning with early biologic agents such as interferons and monoclonal antibodies. The approval of targeted therapies like rituximab and trastuzumab in the 1990s marked a paradigm shift, introducing mechanisms that specifically target cancer cells while sparing healthy tissues. With the advent of recombinant DNA technology, engineered antibodies, antibody-drug conjugates, and immune checkpoint inhibitors became dominant therapeutic classes. These biologics enhanced efficacy in multiple cancer types and offered survival benefits where traditional chemotherapy had limited impact. The rise of immuno-oncology, including PD-1/PD-L1 inhibitors and CAR-T cell therapies, further expanded the biologics landscape. Regulatory frameworks adapted to accelerate approval pathways for breakthrough therapies, encouraging robust R&D investment.
Sources
Primary Research Interviews:
Oncologists
Biotech Researchers
Clinical Trial Managers
Pharmaceutical Executives
Regulatory Specialists
Databases:
NIH Clinical Trials Database
WHO Cancer Statistics
OECD Pharma Data
Magazines:
BioPharma Dive
Nature Biotechnology
Pharmaceutical Technology
Genetic Engineering News
Oncology Times
Journals:
Journal of Clinical Oncology
Cancer Research Journal
Nature Cancer
The Oncologist
Molecular Cancer Therapeutics
Newspapers:
Financial Times (Pharma)
Reuters Healthcare
Bloomberg Life Sciences
The Guardian (Health)
Wall Street Journal
Associations:
American Society of Clinical Oncology
European Society for Medical Oncology
Biotechnology Innovation Organization
International Agency for Research on Cancer
National Cancer Institute
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients