Burkholderia Infections Market Size and Forecast – 2026 – 2033
The global Burkholderia infections market was valued around USD 285.7 Mn in 2026 and is projected to reach about USD 476.3 Mn by 2033, growing at a CAGR of 5.8 %. Growth is driven by rising disease awareness, endemic cases, and R&D for effective therapies.
Global Burkholderia Infections Market Overview
Burkholderia infections are caused by bacteria from the Burkholderia genus, notably Burkholderia pseudomallei, B. cepacia complex, and B. mallei. These pathogens primarily affect immunocompromised individuals and patients with chronic lung diseases such as cystic fibrosis. Infections range from mild respiratory illness to severe pneumonia, septicemia, and melioidosis, a potentially fatal systemic disease. Burkholderia species are intrinsically resistant to many antibiotics, making treatment challenging and prolonged. Transmission occurs through contaminated soil, water, or medical equipment, particularly in tropical and subtropical regions. Rising antimicrobial resistance and improved diagnostic awareness have increased clinical and research focus on Burkholderia infections worldwide.
Key Takeaways
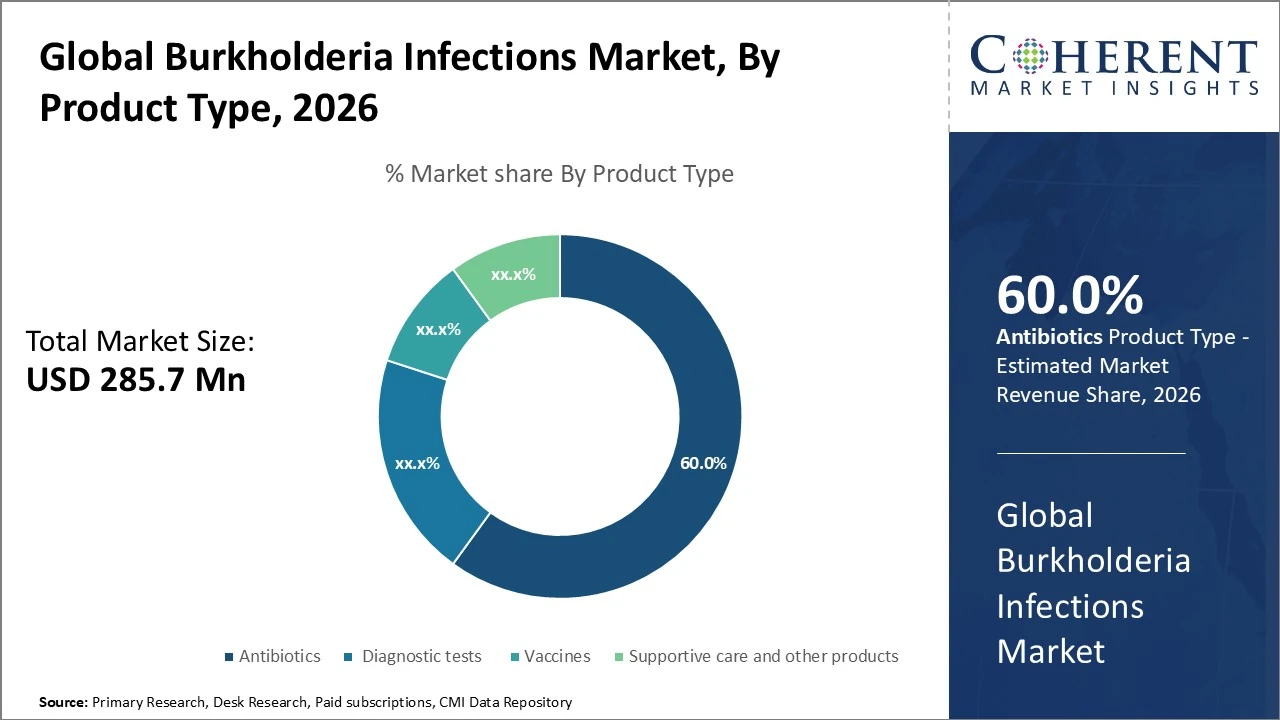
Product Type- is dominated by antibiotics, accounting for approximately 60% of total market share.
Polymerase Chain Reaction hold a significant share, accounting for about 45% of technology.
In the Burkholderia Infections market, hospitals are the dominant end-user, accounting for approximately 60% of market share.
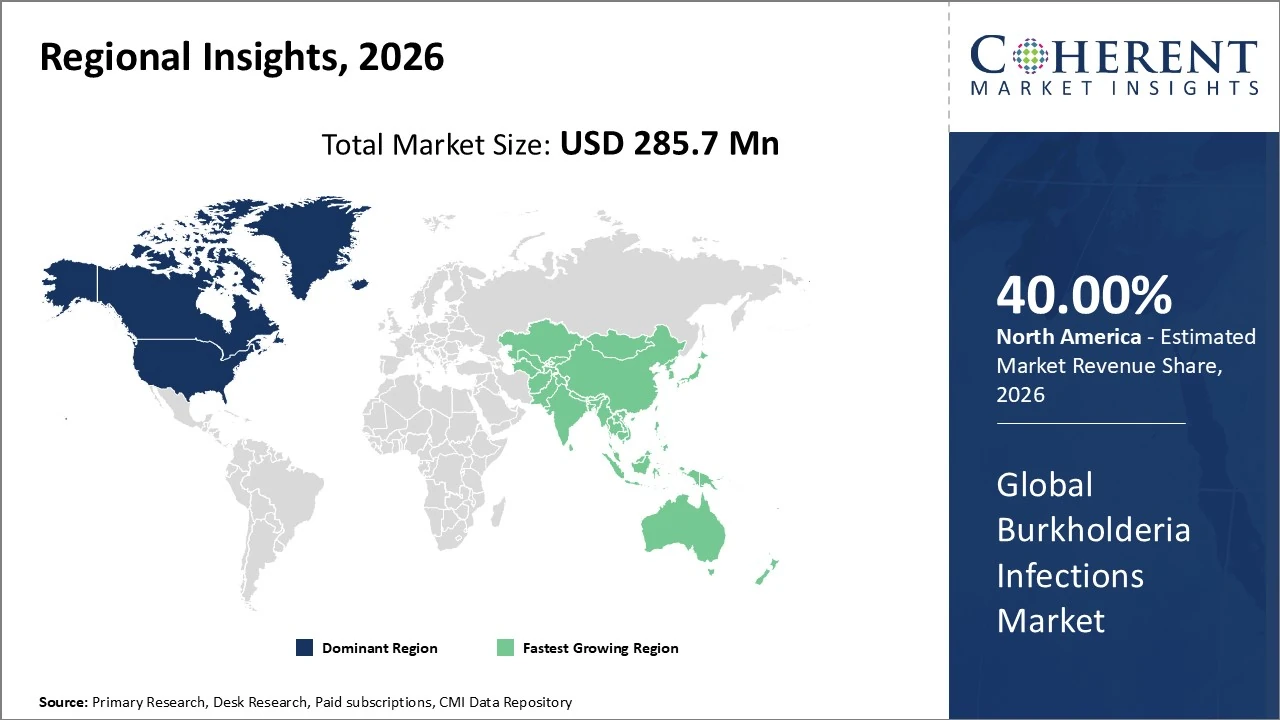
North America dominates the Burkholderia Infections Market, holding about 40% of global revenue share.
Burkholderia Infections Market Segmentation Analysis
To learn more about this report, Download Free Sample
Burkholderia Infections Market Insights, By Product Type
The Burkholderia infections market, by product type, is dominated by antibiotics, accounting for approximately 60% of total market share due to their role as first-line and long-term therapy for melioidosis and related infections. Diagnostic tests represent around 20%, driven by increasing use of PCR, culture, and serological methods for early and accurate detection. Vaccines hold a smaller share of about 10%, reflecting their developmental stage but strong future growth potential. Supportive care and other products contribute roughly 10%, supporting symptom management and treatment outcomes. Overall, treatment products lead, while diagnostics and vaccines gain traction.
Burkholderia Infections Market Insights, By End-User
In the Burkholderia infections market by end-user, hospitals hold the largest share at approximately 60%, as they manage acute and severe cases requiring inpatient treatment and intensive care. Clinics account for around 30%, driven by outpatient care and early diagnosis services. Other end-users contribute roughly 10% of the market. This distribution reflects hospitals’ dominant role in treatment delivery, with clinics growing due to expanded outpatient services and decentralized healthcare access.
Burkholderia Infections Market Trends
Growth in advanced molecular diagnostics (e.g., PCR, rapid tests) improves early detection of Burkholderia species, enhancing treatment outcomes.
Increasing resistance drives demand for novel antibiotics and combination therapies, pushing R&D and market expansion.
Vaccine research and point-of-care technologies are gaining traction, signaling shift toward prevention and decentralized testing in endemic regions.
Burkholderia Infections Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Burkholderia Infections Market Analysis and Trends
In North America, the Burkholderia infections market holds a significant regional share of about 40 % of global revenue, supported by advanced healthcare infrastructure and strong pharmaceutical R&D. The United States leads growth through early adoption of innovative treatments and robust diagnostic capabilities. Market trends include increasing awareness of imported and travel-related cases, enhanced detection technologies, and moderate CAGR expansion driven by improved clinical protocols.
Asia Pacific Burkholderia Infections Market Analysis and Trends
In the Asia Pacific Burkholderia infections market, the region holds a major share of around 35 % of global Burkholderia pseudomallei infections drug revenues, driven by a high disease burden in endemic countries such as Thailand, Malaysia, and northern Australia. Asia Pacific is also the fastest‑growing regional market, with increasing healthcare infrastructure improvements, heightened diagnostic capabilities, and government initiatives enhancing detection and treatment access. Trends include rising incidence of melioidosis cases, expanding pharmaceutical R&D, and heightened awareness among clinicians, supporting sustained market growth through 2033.
Burkholderia Infections Market Outlook for Key Countries
USA Burkholderia Infections Market Analysis and Trends
In the United States Burkholderia infections market, the nation contributes a significant portion of the North American share (~40 %) of the global Burkholderia pseudomallei infections drug segment, driven by strong healthcare infrastructure and research investment. Growth is supported by rising awareness of melioidosis, increased funding for diagnostics and therapeutics, and biodefense concerns since B. pseudomallei is classified as a select agent by U.S. authorities. The market is expanding with novel antibiotics, advanced diagnostics, and public‑private research collaborations. Despite low endemic incidence, travel‑related and emerging cases boost demand for effective clinical management and early detection.
Germany Burkholderia Infections Market Analysis and Trends
In Germany’s Burkholderia infections market, the country represents a notable portion of the broader European Burkholderia pseudomallei infections drug segment, contributing to regional consumption and revenue trends within Europe. Germany benefits from strong healthcare infrastructure, comprehensive diagnostic networks, and rising awareness of rare gram‑negative bacterial infections—such as Burkholderia cepacia complex outbreaks reported by national institutes—which support clinical management and testing uptake. Burkholderia treatment demand is influenced by antimicrobial resistance concerns and advanced hospital settings focusing on rapid diagnostics and effective therapies.
Analyst Opinion
Increasing cases of Burkholderia infections, especially melioidosis in endemic regions, are driving demand for diagnostics and therapies.
Adoption of molecular diagnostics (PCR, NGS) and rapid tests is reshaping early detection and treatment strategies.
Growing resistance to standard antibiotics underscores the need for novel therapies and antimicrobial stewardship programs.
Development of new antibiotics, combination therapies, and preventive measures like vaccines boosts market prospects.
Asia Pacific leads expansion due to endemic disease burden, while developed markets focus on research and advanced care infrastructure.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 285.7 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 5.8% | 2033 Value Projection: | USD 476.3 million |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., GlaxoSmithKline plc, Merck & Co., Inc., Johnson & Johnson, Novartis AG, Sanofi S.A., Roche Holding AG, AstraZeneca plc, and Bayer AG | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Burkholderia Infections Market Growth Factors
The Burkholderia infections market is expanding due to several key growth factors. High prevalence of melioidosis and other Burkholderia-related infections in endemic regions drives demand for diagnostics and treatment. Rising antimicrobial resistance fuels the need for novel antibiotics and combination therapies. Increasing adoption of advanced diagnostic technologies like PCR, serology, and point-of-care tests improves early detection, enhancing treatment outcomes. Government initiatives and funding in infectious disease research support R&D and healthcare infrastructure. Additionally, growing awareness among clinicians and patients, coupled with the emergence of vaccines and preventive solutions, contributes to sustained market growth across regions, particularly in Asia Pacific and North America.
Burkholderia Infections Market Development
In August 2025, DermaRite Industries, LLC expanded the voluntary recall originally initiated on July 16 due to the potential presence of Burkholderia cepacia complex.
Key Players
Leading Companies of the Market
Pfizer Inc.
GlaxoSmithKline plc
Merck & Co.
Novartis AG
Johnson & Johnson
Sanofi S.A.
Roche Holding AG
AstraZeneca plc
Bayer AG
Key players in the Burkholderia infections market include Pfizer Inc., GlaxoSmithKline plc, Merck & Co., Inc., Johnson & Johnson, Novartis AG, Sanofi S.A., Roche Holding AG, AstraZeneca plc, Bayer AG. These companies lead through R&D, diagnostics, antibiotics, vaccines, and strategic collaborations globally.
Burkholderia Infections Market Future Outlook
The future outlook of the Burkholderia infections market is positive, driven by increasing awareness, rising incidence of melioidosis and other Burkholderia-related infections, and the need for effective management of antimicrobial resistance. Advances in molecular diagnostics, rapid testing, and point-of-care solutions are expected to improve early detection and treatment outcomes. Growth will also be fueled by novel antibiotics, combination therapies, and emerging vaccines under development. Asia Pacific is likely to remain the fastest-growing region due to endemic prevalence, while North America and Europe focus on research, advanced healthcare, and imported case management. Overall, innovation and expanded healthcare access will sustain global market growth.
Burkholderia Infections Market Historical Analysis
The Burkholderia infections market historical analysis shows steady growth over the past decade, driven by increasing recognition of diseases such as melioidosis and Burkholderia cepacia complex infections, particularly in endemic regions of Asia Pacific. Early challenges included limited diagnostic capabilities and treatment options, as Burkholderia species exhibit intrinsic resistance to multiple antibiotics. Gradually, adoption of molecular diagnostics, culture-based methods, and improved hospital protocols enhanced detection and management. Pharmaceutical innovation focused on antibiotics and supportive therapies, while awareness campaigns improved clinician knowledge. Historically, North America and Europe contributed through research and imported case management, while Asia Pacific led in treatment volume and market growth.
Sources
Primary Research Interviews:
Infectious Disease Specialists & Clinicians
Pharmaceutical & Biotech Companies
Diagnostic & Laboratory Experts
Hospital & Healthcare Administrators
Databases:
PubMed / Medline
ClinicalTrials.gov
WHO Global Health Observatory
Journals:
Journal of Clinical Microbiology
Clinical Infectious Diseases
Emerging Infectious Diseases
Infection and Immunity
Newspapers:
The Times of India
South China Morning Post
The Guardian
The New York Times
Associations:
American Society for Microbiology (ASM)
Infectious Diseases Society of America (IDSA)
European Society of Clinical Microbiology and Infectious Diseases (ESCMID)
World Health Organization (WHO)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients