Bulimia nervosa is an eating disorder, where a person eat large amount of food in a short duration of time. Eating disorders are usually associated with mental disorders such as depression, anxiety, obsessive compulsive disorder (OCD), and others. There is no specific treatment available for the treatment of bulimia nervosa. Moreover, treatment options such as antidepressants, antipsychotics, exercises, and behavioral therapies are currently available treatment options for bulimia nervosa.
Global Bulimia Nervosa Treatment Market - Impact of the Coronavirus (COVID-19) Pandemic
COVID-19 pandemic is expected to hamper growth of the global bulimia nervosa treatment market during the forecast period. According to the Indian Journal of Medical Sciences, June 6, 2020, the COVID-19 pandemic has affected the conduction of clinical trials due to unavailability of trial site staff, restrictions for travelling, investigational product availability, and others. These challenges are hindrances for developing new treatment for bulimia nervosa.
According to National Center for Biotechnology Information, 24th August 2020, due to COVID-19 pandemic, government imposed lockdown in various parts of the world. This has led to the slowdown of all the businesses, which was the driving force for increasing unemployment among the population. Moreover, lack of social interaction have resulted in increasing cases of anxiety and depression. For instance, according to the Center for Disease Control and Prevention (CDC), 4th April 2020, the cases of depression among the U.S. population has increased by three times during the COVID-19 pandemic as compared to the year 2019, which is one of the causes of bulimia nervosa, among the population.
The global bulimia nervosa treatment market is estimated to be valued at US$ 455.7 Mn in 2020, and is expected to exhibit a CAGR of 5.2% over the forecast period (2020-2027).
Figure 1: Global Bulimia Nervosa Treatment Market Share (%) Analysis, By Treatment Type, 2020
To learn more about this report, Download Free Sample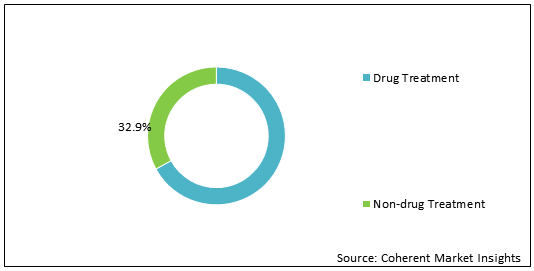
Increasing product launches for the treatment of mental illness among the population is expected to drive growth of the global bulimia nervosa treatment market over the forecast period.
Market players are indulged in product launches, which is expected to drive growth of the global bulimia nervosa treatment market over the forecast period. For instance, in 2019, Lupin Limited launched the generic version of antidepressant Fluoxetine tablets of strengths 10 mg and 20 mg in the U.S. market. Fluoxetine tablets are indicated for the treatment of bulimia nervosa, panic disorder, major depressive disorder, and others.
Bulimia Nervosa Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2020: | US$ 455.7 Mn |
| Historical Data for: | 2017 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 5.2% | 2027 Value Projection: | US$ 684.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Allergan, Inc., Eli Lilly and Company, AstraZeneca plc, Alembic Pharmaceuticals Ltd, Bristol-Myers Squibb Company, GlaxoSmithKline plc, Pfizer Inc., Johnson & Johnson Services, Inc., Dr. Reddy’s Laboratories Limited, Lupin Pharmaceuticals, Inc., Aurobindo Pharma, Zydus Cadila, Apotex Inc., Teva Pharmaceutical Industries Ltd, and Sun Pharmaceutical Industries Ltd |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing product approvals from the regulatory authorities is expected to fuel growth of the market over the forecast period.
Market players are engaged in receiving approvals from the regulatory authorities, which is an important step for launching the product in the market. For instance, on 9th April 2020, Aurobindo Pharma received the U.S. Food and Drug Administration (U.S. FDA) approval for its generic anti-depressant Fluoxetine tablets, which will allow the manufacture and marketing of the Fluoxetine tablets of strengths 10 mg and 20 mg. The product is the generic version of Eli Lily and Company’s Prozac tablets.
Global Bulimia Nervosa Treatment Market – Restraints
Lack of proper reimbursement policies for the treatment of eating disorders is expected to restrain growth of the global bulimia nervosa treatment market over the forecast period. For instance, according to the National Eating Disorders Associations, per year, in the U.S., the total treatment cost for the eating disorders is valued at US$ 30,000 per patient. Even in the presence of insurance, the patients has to shell out more money for the treatment. Moreover, according to the same source, in the U.S., there were approximately 54,000 emergency department admissions and 23,500 in-patient hospitalizations visits due to eating disorders in the year span of 2018 – 2019, costing around US$ 29 million and US$ 209 million respectively.
Furthermore, lack of drugs specifically for the treatment of bulimia nervosa treatment and lack of awareness regarding the treatment options among the population are the factors that are expected to restrain growth of the global bulimia nervosa treatment market over the forecast period.
Global Bulimia Nervosa Treatment Market – Regional Analysis
North America is expected to hold dominant position in the global bulimia nervosa treatment market over the forecast period, owing to the increasing prevalence of the eating disorders in the region. For instance, according to the article published in the Biological Psychiatry Segment of Elsevier Journal: 2018, in the U.S., 36,309 adults suffering from eating disorders were recruited for studying the prevalence of bulimia nervosa among the population. It was estimated that, out of the total adults studied, 0.80%, 0.28%, and 0.85% of the U.S. adults were suffering from anorexia nervosa, bulimia nervosa, and binge eating disorder respectively.
Europe is also an emerging bulimia nervosa treatment market, owing to the increasing research activities for developing new treatment for the eating disorder. For instance, on 2nd March 2020, National Institute for Health Research (NIHR), in partnership with Eating Disorders Genetics Initiative (EDGI), recruited 10,000 people for studying eating disorder especially bulimia nervosa, which will help in developing novel treatment options to improve the lives of the patients suffering from eating disorder.
Figure 2: Global Bulimia Nervosa Treatment Market Share (US$ Mn), by Region, 2020
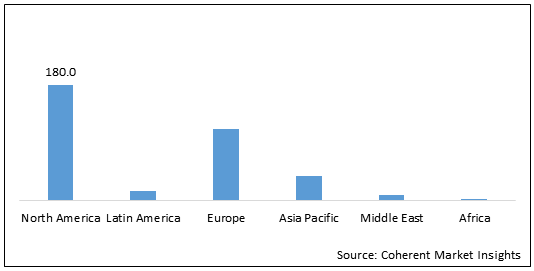
To learn more about this report, Download Free Sample
Global Bulimia Nervosa Treatment Market – Competitive Landscape
Some of the key players operating in the global bulimia nervosa treatment market include Allergan, Inc., Eli Lilly and Company, AstraZeneca plc, Alembic Pharmaceuticals Ltd, Bristol-Myers Squibb Company, GlaxoSmithKline plc, Pfizer Inc., Johnson & Johnson Services, Inc., Dr. Reddy’s Laboratories Limited, Lupin Pharmaceuticals, Inc., Aurobindo Pharma, Zydus Cadila, Apotex Inc., Teva Pharmaceutical Industries Ltd, and Sun Pharmaceutical Industries Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients