Budesonide is a medication that is used to prevent symptoms of asthma. It is a corticosteroid or steroid (cortisone-like medicine). The medication prevents inflammation in the lungs to decrease the severity of asthma attack. Inhaled budesonide may be used with other asthma medicines such as bronchodilators, which are also used to open up narrowed breathing passages in the lungs. Budesonide is available in several forms such as powders, suspensions, and tablets. In powder form, the medication can be inhaled by mouth using an inhaler. It can also be used as suspension to inhale by mouth using a special jet nebulizer (machine that turns medication into a mist that can be inhaled). Some of the trade names under which budesonide is sold are Pulmicort, Rhinocort, and Entocort.
Global budesonide inhaler market size was valued at US$ 6,899.6 Million in 2022 and is expected to witness a CAGR of 6.0% over the forecast period (2022 – 2030).
Figure 1.Global Budesonide Inhaler Market Share (%), by Product Type, 2022
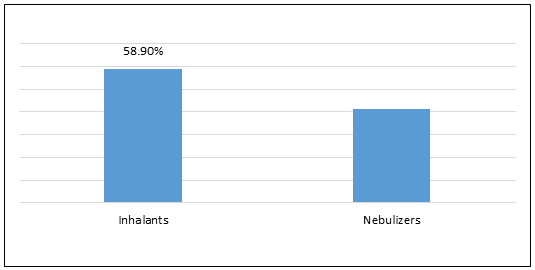
To learn more about this report, Download Free Sample
Increasing number of launches in generic version of budesonide inhaler is expected to drive growth of the budesonide inhaler market over the forecast period.
Increasing number of launches in generic version of budesonide inhaler is a major driving factor for growth of the budesonide inhaler market growth. For instance, in April 2020, Cipla Limited, a pharmaceutical company, announced that it received final approval for its Abbreviated New Drug Application (ANDA) for Albuterol Sulfate Inhalation Aerosol 90mcg (base)/actuation, from the U.S. Food and Drug Administration (FDA). Cipla’s Albuterol Sulfate Inhalation Aerosol 90mcg (base)/actuation, is AB-rated (a product approved by a regulatory agency that has determined the product to be bioequivalent to an existing or already-approved product identified as an Approved Product Concept.) generic therapeutic equivalent version of Merck Sharp & Dohme Corp’s Proventil HFA Inhalation Aerosol. It is used for treatment of acute episodes of bronchospasm or prevention of asthmatic symptoms.
Figure 2. Global Budesonide Inhaler Market Share (%), by Region, 2022
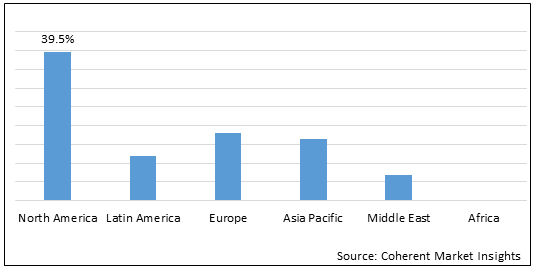
To learn more about this report, Download Free Sample
Increasing prevalence of chronic obstructive pulmonary disease and asthma in the U.S. is expected to boost the growth of global budesonide inhaler over the forecast period.
Increasing prevalence of chronic obstructive pulmonary disease and asthma in the U.S. is expected to boost the growth of budesonide inhaler market. For instance, on August 19, 2022, according to the reports published by Center Of Disease Control And Prevention, National Center of Health Statistics published a 2020 report which stated that, 5.0% of the U.S. population have been diagnosed with chronic obstructive pulmonary disease (COPD), emphysema, or chronic bronchitis, 4.1% of visits to office-based physicians with (COPD) chronic obstructive pulmonary disease indicated on the medical record in 2018.
Moreover, according to the same source, on August 16, 2022, according to the 2020 report, percent of adults aged 18 and over who currently have asthma is 8.4% and 5.8% of children under age 18 years who currently have asthma in the U.S. Whereas, 5.8 million was the annual number of office visits for asthma in 2020.
Budesonide Inhaler Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 6,899.6 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 6.0% | 2030 Value Projection: | US$ 10,993.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Cipla Limited, Lupin Limited, Cosmo Pharmaceuticals, Takeda Pharmaceutical Company Ltd., Manus Aktteva Biopharma LLP, Abbott Laboratories, Lunan Better Pharmaceutical, Novartis International AG (Sandoz), Mylan N.V., Skyepharma, AstraZeneca Plc., Chiesi Farmaceutici S.p.A, Orion Corporation, Santarus Inc., Synmosa Biopharma Corporation, and Shanghai Sine Pharmaceutical Laboratories Co. Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Budesonide Inhaler Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
The sudden outbreak of COVID-19 has bought the world to a standstill. The whole world is fighting this pandemic with increased burden on hospitals and healthcare professionals. In April, 2020, University of Oxford, published a report on Asthma drug budesonide shortens recovery time in non-hospitalized patients with COVID-19, which reported that early treatment with inhaled budesonide shortens recovery time by a median of three days in patients with COVID-19 who are at higher risk of more severe illness, whereas Inhaled budesonide is a safe, relatively inexpensive and readily available corticosteroid commonly used around the world in inhalers to treat asthma and chronic obstructive pulmonary disease. The results showed that estimated median time to self-reported recovery for inhaled budesonide was 3.011 days shorter compared to usual care.
Thus, impact of covid-19 has driven the growth of global budesonide inhaler market during the pandemic.
Global Budesonide Inhaler Market: Key Developments
Budesonide Inhaler Market Restraints
The major restraining factor for budesonide inhaler market growth is the side effects shown by budesonide such as
Moreover, product recall is the major factor which hinders the growth of global budesonide inhaler market over the forecast period. For instance, in September 2020, Perrigo Company plc, a Pharmaceutical company, announced a voluntary U.S. nationwide recall of albuterol sulfate inhalation aerosol to the retail level after halting production and distribution. The actions were been taken out of an abundance of caution as a result of complaints that some units may not dispense due to clogging. Perrigo's generic albuterol sulfate inhalation aerosol was developed in partnership with and manufactured by Catalent Pharma Solutions, a pharmaceutical company.
Key Players
Key players operating in the global budesonide inhaler market include Pfizer Inc., Cipla Limited, Lupin Limited, Cosmo Pharmaceuticals, Takeda Pharmaceutical Company Ltd., Manus Aktteva Biopharma LLP, Abbott Laboratories, Lunan Better Pharmaceutical, Novartis International AG (Sandoz), Mylan N.V., Skyepharma, AstraZeneca Plc., Chiesi Farmaceutici S.p.A, Orion Corporation, Santarus Inc., Synmosa Biopharma Corporation, and Shanghai Sine Pharmaceutical Laboratories Co. Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients