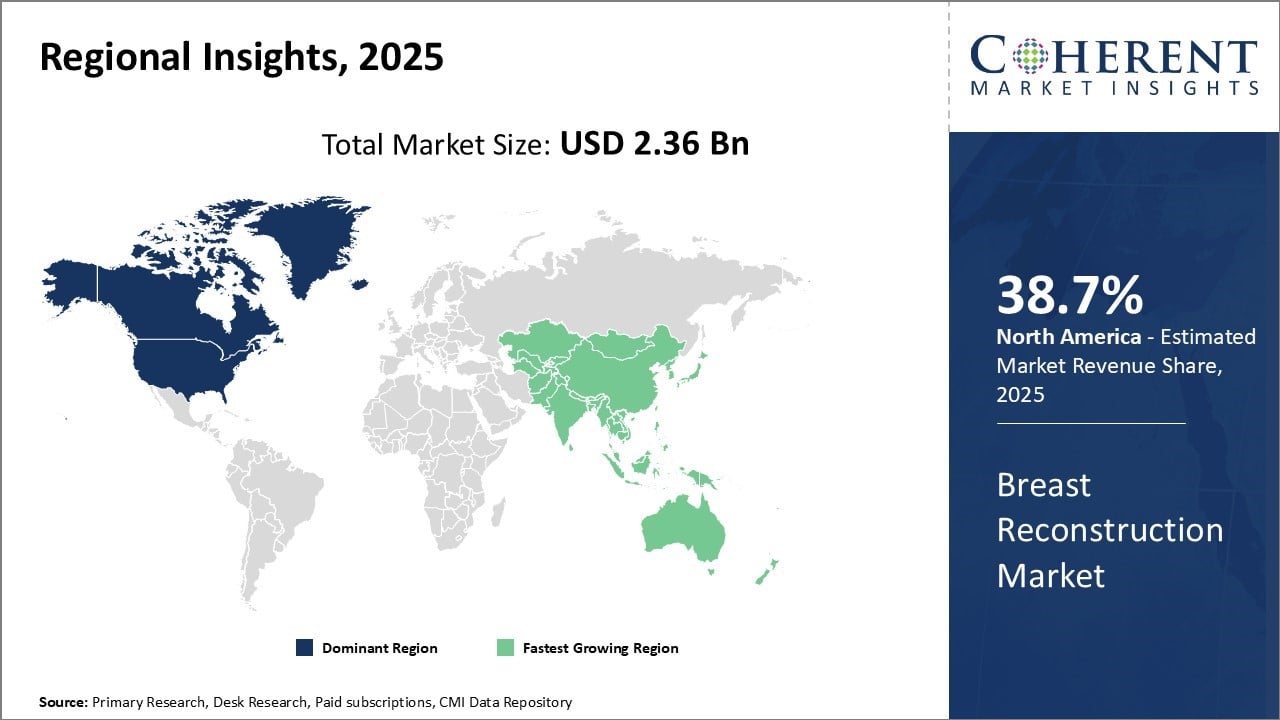
The Global Breast Reconstruction Market size is estimated to be valued at USD 2.36 Bn in 2025 and is expected to reach USD 3.81 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.1% from 2025 to 2032.
To learn more about this report, Download Free Sample
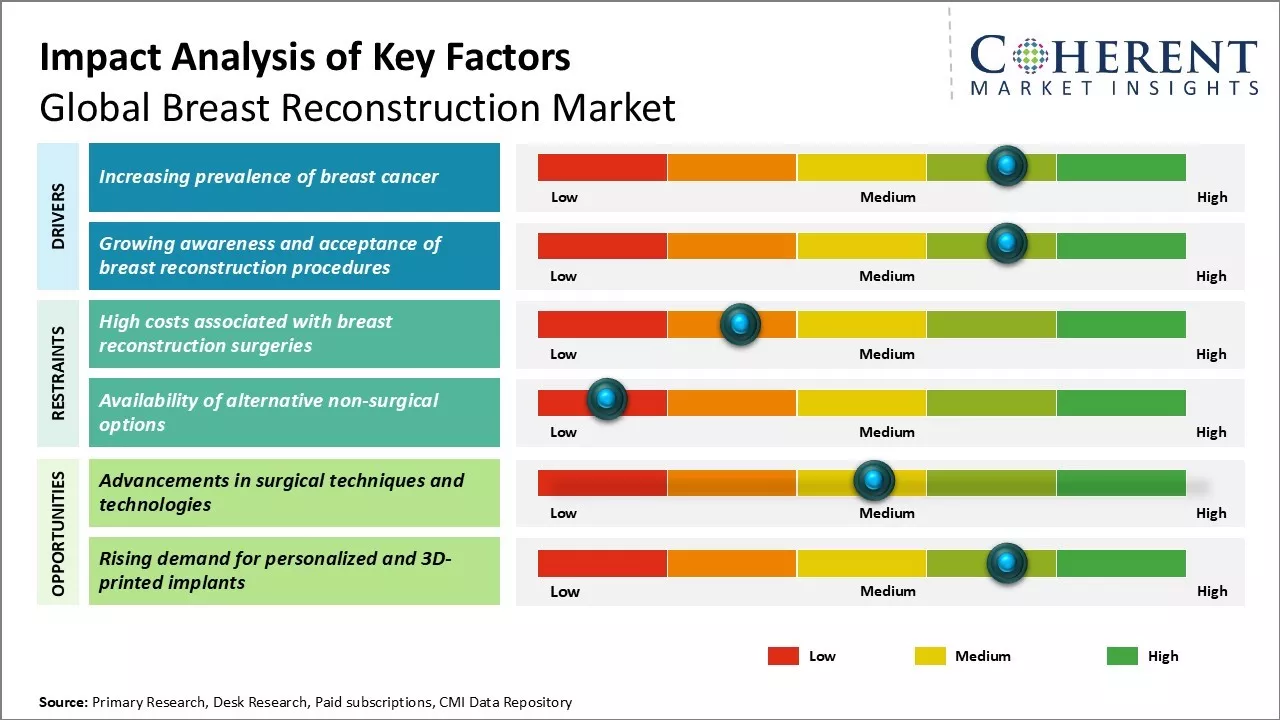
The breast-reconstruction market growth is expanding mainly due to more women are entering adulthood and because doctors keep spotting more breast-cancer cases around the world. On top of that, new implants keep hitting the shelves and a steady stream of U.S. FDA nods for these products will probably fuel sales even more over the next few years.
|
Event |
Description and Impact |
|
Regulatory Updates on Implant Safety |
|
|
U.S.-China Trade Tariffs |
|
|
Technological Advancements |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of product type, implants grab the largest share of the breast-reconstruction market value, about 42.6% in 2025, because many women choose them for their ease and ability to look more natural afterward. The option is especially appealing because doctors don’t need to take tissue from another body area. That small difference spares patients extra scars and the longer healing that often comes from a donor site.
When it comes to breast implants, industry observers now see the round model staying on top and grabbing an estimated 36.6 percent of all sales by 2025, mainly because many women still ask for that rounded, youthful look. Its smooth, even curve so closely copies a naturally perky breast that plastic surgeons often point to it as the gold standard for giving volume up high. Since the lower half stays noticeably fuller, the implant keeps its forward projection even years later, reducing the chance of the breast drooping. For clients after reconstruction or simply upgrade surgery, that steady, feminine outline-the one everyone expects-is practically what every cut and stitch is designed to deliver.
In 2025, immediate breast reconstruction acquires nearly fifty-one point six percent-simply because it tackles most of the collateral damage breast-cancer care can leave behind. By inserting tissue expanders or implants right at the mastectomy bench, surgeons spare their patients the sudden loss of shape, the dip in self-worth, and the worry and sadness that often follow when one breast disappears
To learn more about this report, Download Free Sample
North America dominates in the breast reconstruction market demand with an estimated share of 38.7% in 2025. This can be attributed to factors such as the presence of advanced healthcare infrastructure, high healthcare spending, and favorable reimbursement policies. The region is also home to major reconstructive surgery providers and medical technology companies.
The Asia-Pacific region is growing the quickest, spurred by better access to health care, a surge in medical tourism, and rising public awareness of new treatments. China, India, and Japan stand out as major markets where adoption is on the upswing. In response, top international companies are widening their presence by teaming up with local hospitals and training surgeons.
During the forecast period, the U.S. will probably stay at the front, pulled along by high breast cancer rates, an increasing hunger for breast implants, and a health-care system that already has all the pieces in place. To illustrate, the American Society of Plastic Surgeons reported that, in 2020, breast augmentation still sat among the five busiest cosmetic surgeries performed in the country.
Canada is quickly carving out a prominent place in the breast-reconstruction field, spurred by a steady stream of new product approvals and a sharper national focus on breast-cancer care. A telling example came in March 2022, when Health Canada cleared Sientra Inc.s High-Strength Cohesive HSC and HSC silicone-gel implants, giving the market fresh momentum. Growing breast-cancer rates, paired with supportive regulators and ever more active manufacturers, are cementing this upward trend across the country.
Germany is a dominant player in the breast reconstruction and aesthetics market due to its robust healthcare infrastructure and continuous innovation. POLYTECH, a Germany-based leader in breast implants, introduced Opticon Plus in September 2025, further solidifying the country’s market leadership. The implant offers advanced anatomical options and innovative surface technologies like MESMO and Microthane, meeting the growing global demand for natural aesthetics. Germany’s strong R&D focus and ability to cater to diverse surgical needs make it a global hub for breast aesthetics advancements.
Japan is a dominant market in breast aesthetics and reconstruction due to its innovative approach and alignment with cultural preferences. For instance, in April 2023, Establishment Labs Holdings Inc. launched Mia Femtech in Japan, a revolutionary breast aesthetics solution. Mia offers a minimally invasive, 15-minute procedure without general anesthesia, delivering natural, discreet results with minimal downtime.
To learn more about this report, Download Free Sample
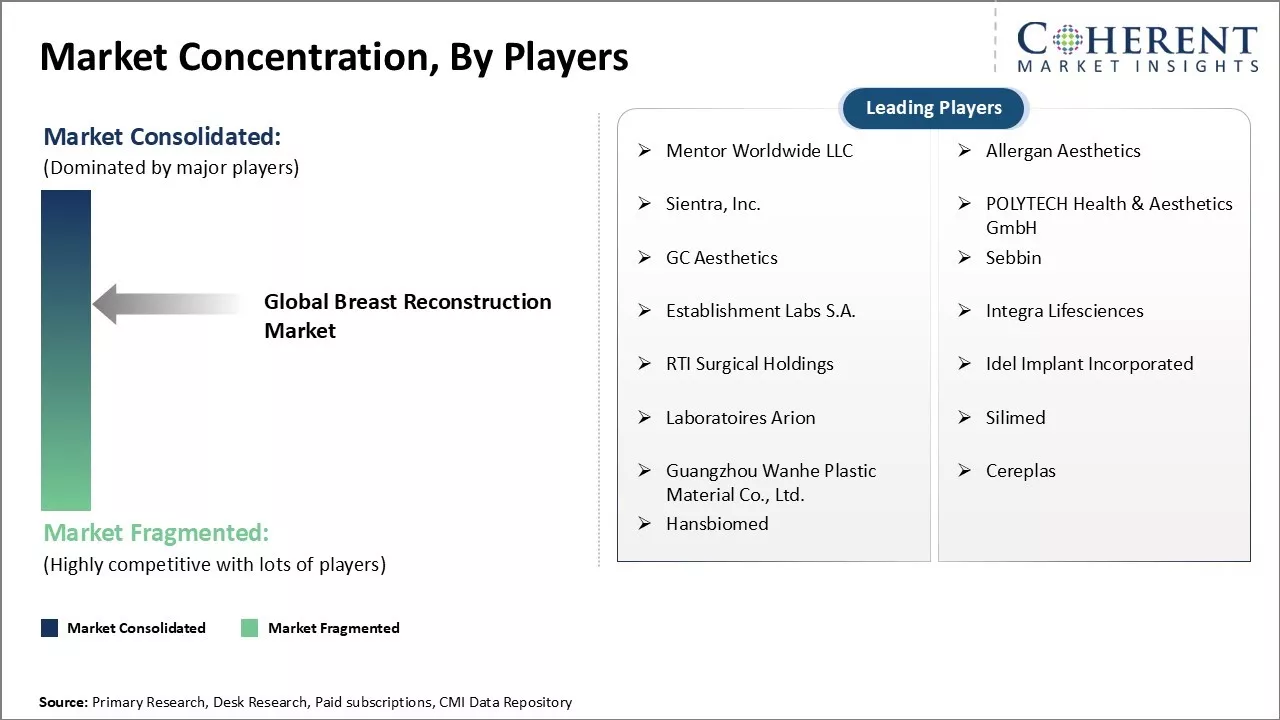
Established Players: Leading firms in the worldwide breast-reconstruction arena pour huge energy into R and D, chasing high-performing breakthroughs. As of March 2024, giants such as Johnson & Johnson set aside over 10 percent of yearly income for this purpose. Their labs, stuffed with expert scientists and engineers, push forward the next wave of tissue expanders, implants and other vital tools.
Mid-Level Players: Mid-tier brands zero in on solid, wallet-friendly goods that stay within reach of most budgets. They reach price-conscious buyers in emerging markets with lean, cost-smart plans. Tactics include choosing less expensive raw stock, fine-tuning production lines and farming out secondary work. Such discipline lets these firms deliver real value while keeping quality intact.
Small-Scale Players: Smaller outfits survive-and thrive-by serving narrow niches that the big names overlook. Some dedicate themselves to fresh answers in fields like nerve-repair surgery. Others use 3D modeling and printing to create one-off implants custom-made for each patient.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.36 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.1% | 2032 Value Projection: | USD 3.81 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Mentor Worldwide LLC , Allergan Aesthetics , Sientra, Inc., POLYTECH Health & Aesthetics GmbH, GC Aesthetics, Sebbin, Establishment Labs S.A., Integra Lifesciences, RTI Surgical Holdings, Idel Implant Incorporated, Laboratoires Arion, Silimed, Guangzhou Wanhe Plastic Material Co., Ltd., Cereplas, and Hansbiomed |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The global prevalence of breast cancer has been rising steadily over the past few decades. According to WHO estimates released in March 2024, around 2 million new cases of breast cancer are diagnosed each year worldwide. Early detection through screening programs has contributed to higher rate of diagnosis, however, even accounting for this, there has been a consistent rise in the incidence of breast cancer cases. Breast cancer prevalence is rising due to Western lifestyle adoption, aging populations, and hormonal and reproductive factors.
There have been significant advancements in recent years regarding the techniques and technologies available for breast reconstruction surgeries. New procedures like deep inferior epigastric perforator flap reconstruction. are allowing women to regain breast tissue using their own body tissues rather than implants. This provides a more natural look and feel but does require highly specialized microsurgery skills. Additionally, surgeons now have access to 3D modeling and planning tools which can help tailor reconstruction plans to each individual patient's anatomy.
The breast reconstruction market has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Medical Devices Stakeholder |
Opportunities Due to Breast Reconstruction Industry Growth |
|
Retail Pharmacies |
Offering medical devices for home use, such as blood glucose monitors and digital thermometers, expanding product offerings and customer care services. |
|
Medical Device Manufacturers |
Expansion of product lines to include innovative devices such as wearables, smart implants, and AI-driven diagnostic tools. |
|
Healthcare Providers |
Opportunities to adopt and integrate cutting-edge medical devices into patient care, improving outcomes and expanding service offerings. |
|
Biotech Firms |
Collaborating on the development of combination products, such as drug-device combinations, offering new treatment options and expanding markets. |
|
Regulatory Affairs Specialists |
Growing demand for expertise in navigating the complex regulatory landscape for medical device approvals, ensuring compliance with global standards. |
|
Supply Chain and Logistics Providers |
Managing the distribution of sensitive and high-value medical devices, including ensuring compliance with medical regulations and standards. |
|
Medical Device Consultants |
Providing strategic guidance on product development, regulatory compliance, and market entry for medical device companies. |
|
Healthcare Training Institutions |
Expanding training programs to include the use of advanced medical devices, preparing healthcare professionals for the latest technological advancements. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients