Breast Cancer Therapeutics Market Size and Forecast – 2026 – 2033
The Global Breast Cancer Therapeutics Market size is estimated to be valued at USD 15.80 billion in 2026 and is expected to reach USD 28.40 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 8.6% from 2026 to 2033.
Global Breast Cancer Therapeutics Market Overview
The breast cancer therapeutics market comprises a range of treatments designed to target and manage different subtypes of breast cancer. Key products include hormone therapies such as tamoxifen and aromatase inhibitors, targeted therapies like HER2 inhibitors (trastuzumab, pertuzumab), CDK4/6 inhibitors, and PARP inhibitors. Chemotherapy agents, including anthracyclines and taxanes, remain widely used for aggressive cancers. Immunotherapies and novel antibody-drug conjugates are emerging, offering precision-targeted treatment with reduced systemic toxicity. Supportive care drugs, including antiemetics and bone-modifying agents, complement primary therapies. The market is driven by innovations in personalized medicine, biomarker-based treatments, and combination therapies to improve efficacy and patient outcomes.
Key Takeaways
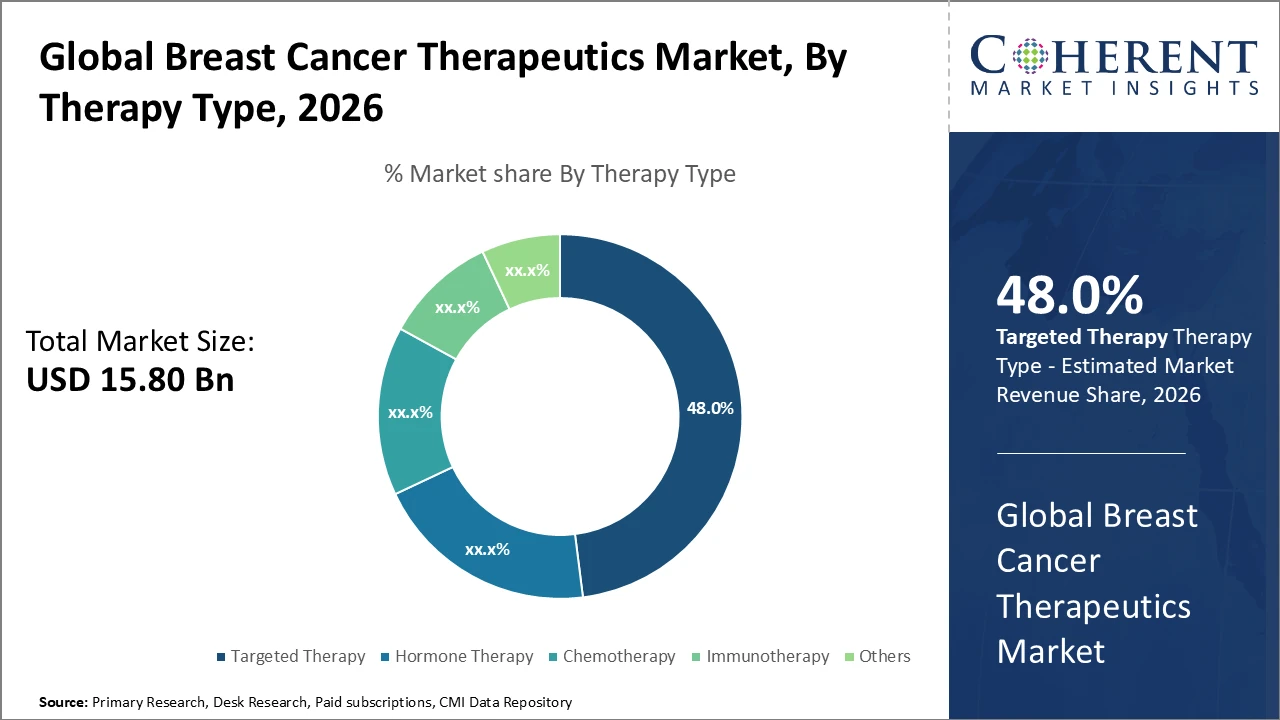
Targeted therapy dominates the market with a 48% share, driven by the successful implementation of precision oncology drugs
Hormone therapy and chemotherapy remain essential treatment options but face growing challenges from evolving therapeutic alternatives
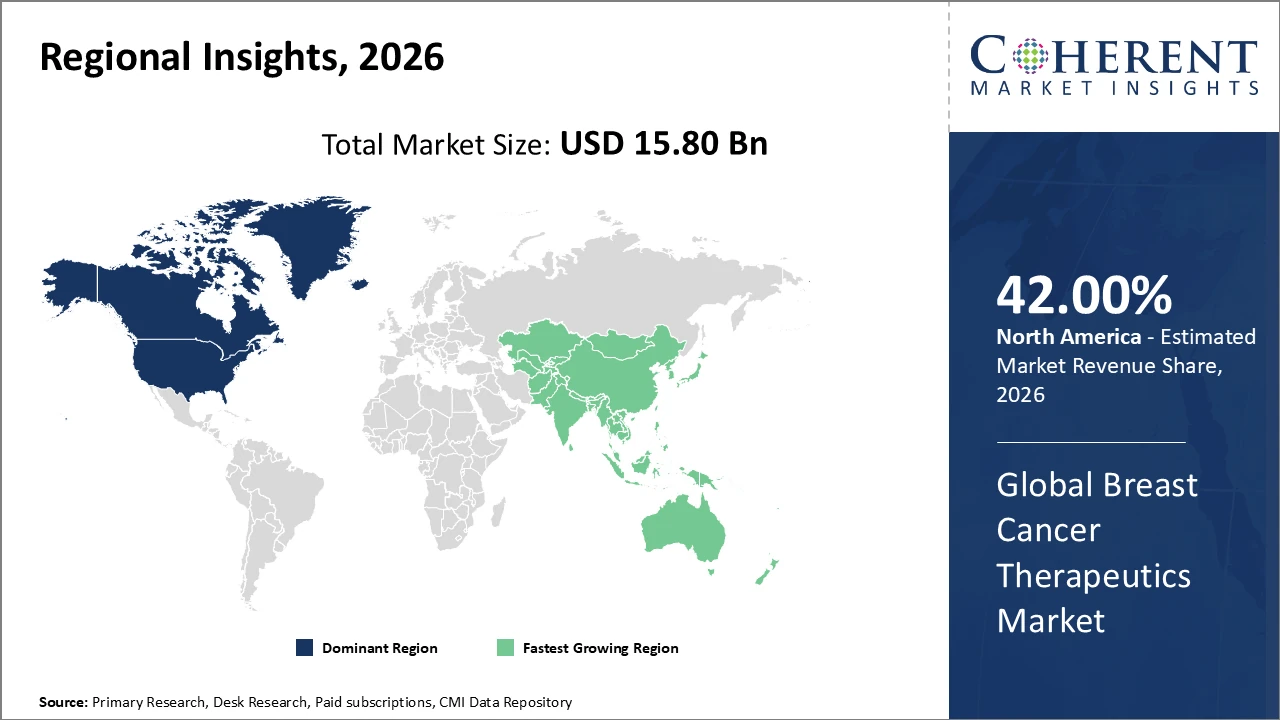
North America holds the largest market share, supported by a consolidated healthcare infrastructure and early adoption of novel therapeutics
Asia Pacific is the fastest-growing regional market, with a CAGR exceeding 10%, driven by rising healthcare investments and increasing patient awareness
Europe shows steady and robust growth, mainly due to regulatory harmonization and innovation-friendly healthcare policies
Breast Cancer Therapeutics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Breast Cancer Therapeutics Market Insights, By Therapy Type
Targeted therapy remains the leading driver in the breast cancer therapeutics market, supported by widespread adoption of monoclonal antibodies such as trastuzumab and pertuzumab, which deliver superior outcomes in HER2-positive breast cancer. Pipeline innovations, including upcoming antibody-drug conjugates, are expected to further boost revenues. Immunotherapy shows the fastest growth, driven by immune checkpoint inhibitors and combination therapies addressing treatment-resistant cancers, with multiple FDA breakthrough designations in 2025 highlighting its potential. Endocrine therapy continues to treat hormone-receptor-positive cases through estrogen receptor modulators and aromatase inhibitors. Chemotherapy faces slower growth due to toxicity, while emerging agents like PARP inhibitors expand the “Others” subsegment.
Breast Cancer Therapeutics Market Insights, By Cancer Stage
Early-stage breast cancer dominates the market, driven by widespread screening and early diagnosis initiatives worldwide. Therapeutics in this segment benefit from adjuvant and neoadjuvant treatment protocols that improve survival rates, while advances in endocrine and targeted therapies help minimize recurrence risk. Less invasive regimens enhance patient compliance and outcomes, sustaining steady demand. Metastatic breast cancer represents the fastest-growing subsegment, as novel immunotherapies and combination treatments address complex needs in advanced disease, supported by increased investment and clinical trial activity. Recurrent breast cancer therapies focus on personalized interventions targeting therapy resistance, with emerging agents addressing specific genetic mutations to maintain market relevance.
Breast Cancer Therapeutics Market Insights, By Distribution Channel
Hospital pharmacies dominate the market due to centralized administration of oncology drugs, supported by integrated care models and strong clinical trial networks that drive higher drug adoption. Specialty clinics are the fastest-growing segment, benefiting from their oncology-focused approach and ability to deliver advanced and personalized therapies. Retail pharmacies continue to play a steady role by supporting outpatient prescription refills and distributing generic treatments. Online pharmacies are rapidly emerging, particularly after the pandemic, enhancing patient convenience and market reach through digital platforms. The others segment, including government institutions and charitable clinics, contributes by providing subsidized therapies, collectively improving access and market penetration.
Breast Cancer Therapeutics Market Trends
Integration of artificial intelligence (AI) in drug discovery and patient treatment planning is accelerating the development of personalized breast cancer therapies in 2025 and 2026.
Advances in multi-omics profiling have improved understanding of tumor heterogeneity, supporting the growth of combination therapy regimens, especially in metastatic breast cancer.
The market is shifting toward patient-centric oral and subcutaneous administration routes, with oral CDK4/6 inhibitors capturing over 25% of revenue share in 2026.
Increased adoption of immuno-oncology agents, reflected in rising clinical trial activity, signals a paradigm shift aimed at achieving durable responses in treatment-resistant breast cancer.
Breast Cancer Therapeutics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Breast Cancer Therapeutics Market Analysis and Trends
North America leads the breast cancer therapeutics market due to its well-established healthcare infrastructure, strong research and development capabilities, and efficient regulatory frameworks that enable rapid approval of innovative therapies. The United States alone contributes over major revenue, driven by extensive clinical trial activity, early adoption of advanced treatments, and high levels of patient and physician awareness. Favorable reimbursement policies further support market growth. Major pharmaceutical players such as Roche and Pfizer have successfully capitalized on this environment, strengthening their market position through continuous innovation, robust product pipelines, and strategic collaborations.
Asia Pacific Breast Cancer Therapeutics Market Analysis and Trends
Asia Pacific represents the fastest-growing breast cancer therapeutics market, with a CAGR exceeding 10%, fueled by rising healthcare expenditure, increasing breast cancer incidence, and improving access to advanced treatment options. Government-led initiatives in countries such as China and India have strengthened screening and early detection programs, leading to higher diagnosis rates and greater therapeutic adoption. Additionally, the growing presence of local generic and biosimilar manufacturers has supported market expansion by offering cost-effective alternatives. Competitive pricing and expanding healthcare infrastructure continue to broaden patient access, positioning Asia Pacific as a key growth engine in the global market.
Breast Cancer Therapeutics Market Outlook for Key Countries
USA Breast Cancer Therapeutics Market Analysis and Trends
The U.S. breast cancer therapeutics market is driven by early adoption of advanced treatment modalities and substantial investments in oncology research and development. Strong regulatory support, including FDA approvals of next-generation immunotherapies between 2024 and 2026, has significantly expanded available treatment options and improved patient survival outcomes. Leading pharmaceutical companies such as AstraZeneca and Bristol-Myers Squibb have strengthened their market presence by leveraging accelerated approval pathways and forming strategic collaborations with academic and research institutions. Additionally, the country’s well-developed healthcare infrastructure and broad insurance coverage continue to reinforce its leading position in market revenue and overall business growth.
Germany Breast Cancer Therapeutics Market Analysis and Trends
Germany’s breast cancer therapeutics market is driven by a strong healthcare system, advanced diagnostic capabilities, and high adoption of innovative treatment options. Favorable reimbursement policies and universal health coverage support broad patient access to targeted therapies, immunotherapies, and hormone treatments. The country benefits from a well-established regulatory framework aligned with the European Medicines Agency, enabling timely introduction of novel drugs. Growing emphasis on personalized medicine and biomarker-based therapies is shaping treatment trends. Additionally, active clinical research, collaborations between pharmaceutical companies and academic institutions, and increasing awareness programs continue to support steady market growth and therapeutic innovation across Germany.
Analyst Opinion
Adoption of targeted therapies such as CDK4/6 and PARP inhibitors has increased significantly due to their precision in treating specific breast cancer subtypes. The introduction of CDK4/6 inhibitors resulted in a 22% improvement in progression-free survival among hormone receptor-positive patients in 2025, strongly boosting market revenue and overall market share.
Immunotherapy is expanding beyond metastatic settings into early-stage treatment. In 2024, approval of immunotherapies for neoadjuvant use increased patient eligibility by 18%, driving growth in both market size and share and signaling a shift in demand-driven market dynamics.
Cost-efficiency pressures in emerging economies are reshaping pricing models. Strategic price adjustments across Asia Pacific in 2026 improved therapy accessibility by 25%, increasing imports and expanding regional market scope through supply-side optimization.
Combination therapeutics integrating chemotherapy, hormone therapy, and biologics are gaining traction. Clinical trials in 2025 showed up to a 30% improvement in overall survival, supporting higher market revenue and signaling a transformative phase in therapeutic development.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 15.80 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.6% | 2033 Value Projection: | USD 28.40 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Roche, Pfizer, Novartis, AstraZeneca, Ely Lilly and Company, Sanofi, Johnson & Johnson, Merck & Co., Amgen, GlaxoSmithKline | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Breast Cancer Therapeutics Market Growth Factors
The global rise in breast cancer incidence, especially in developing regions, continues to drive market growth. In 2026, breast cancer accounted for over 25% of newly diagnosed cancer cases among women, significantly increasing demand for effective therapeutics. The integration of precision medicine, combining genomic profiling with tailored treatment regimens, has improved clinical outcomes and patient adherence, directly boosting market revenue. Government initiatives promoting cancer awareness and increased funding for therapeutic innovation further support business expansion. Ongoing advancements in combination therapies targeting resistant subtypes, along with expanding healthcare infrastructure—particularly across Asia Pacific—are enhancing patient access and sustaining long-term market growth.
Breast Cancer Therapeutics Market Development
In December 2025, the FDA approved AstraZeneca and Daiichi Sankyo’s Enhertu (trastuzumab deruxtecan), in combination with pertuzumab, as a first-line treatment for adults with unresectable or metastatic HER2-positive breast cancer, as confirmed by an FDA-approved diagnostic test.
Key Players
Leading Companies of the Market
Roche
Pfizer
Novartis
AstraZeneca
Ali Lilly and Company
Sanofi
Johnson & Johnson
Merck & Co.
Amgen
GlaxoSmithKline
Several leading companies have leveraged strategic partnerships and acquisitions to enhance portfolio diversity and expand global presence. In 2025, Roche acquired genomic testing firms, enabling integrated theranostic approaches that personalized treatment selection. This strategy contributed to a 15% increase in regional revenue from targeted therapies, highlighting the financial and clinical impact of such acquisitions. Similarly, Pfizer prioritized accelerating clinical trials for innovative immunotherapeutics, which led to FDA approvals and expanded its breast cancer therapeutics pipeline by 25% in 2024, strengthening its competitive positioning and reinforcing its capacity to deliver next-generation treatment options worldwide.
Breast Cancer Therapeutics Market Future Outlook
The breast cancer therapeutics market is expected to experience robust growth driven by continued innovation in targeted therapies, immunotherapies, and combination regimens. Advances in precision medicine, including genomic profiling and biomarker-driven treatments, will enhance patient-specific therapy and improve outcomes. Emerging markets, particularly in Asia Pacific, are projected to expand rapidly due to rising healthcare investment, improved infrastructure, and growing awareness programs. Ongoing clinical trials and regulatory support for next-generation therapeutics will further broaden treatment options. Strategic collaborations, acquisitions, and digital healthcare integration are likely to optimize market reach, patient access, and overall revenue, shaping a dynamic and competitive future landscape.
Breast Cancer Therapeutics Market Historical Analysis
The breast cancer therapeutics market has historically experienced steady growth, driven by the rising global prevalence of breast cancer and increasing awareness of early detection and treatment. Traditional therapies, including chemotherapy, hormone therapy, and surgery, formed the backbone of treatment, while targeted therapies and biologics began gaining prominence in the 2010s. Early adoption in developed regions like North America and Europe was supported by advanced healthcare infrastructure, robust R&D, and favorable regulatory frameworks. Over time, innovations such as CDK4/6 inhibitors, PARP inhibitors, and monoclonal antibodies expanded treatment options, improved patient outcomes, and gradually shifted market dynamics toward precision and personalized medicine.
Sources
Primary Research Interviews:
Oncologists and oncology surgeons
Clinical researchers in breast cancer
Pharmacologists specializing in oncology drugs
Hospital pharmacy managers
Patient advocacy groups for breast cancer
Databases:
National Cancer Institute (NCI) SEER Database
World Health Organization (WHO) Cancer Statistics
European Cancer Information System (ECIS)
IQVIA Oncology Data
Magazines:
Cancer Therapy Advisor
Oncology Times
Targeted Oncology
Pharmaceutical Technology
Journals:
The Breast
Cancer Research
Journal of Clinical Oncology
Breast Cancer Research and Treatment
The Lancet Oncology
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
American Cancer Society (ACS)
European Society for Medical Oncology (ESMO)
Breast Cancer Research Foundation (BCRF)
National Cancer Institute (NCI)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients