Bladder Cancer Therapeutics Market Size and Forecast – 2026 – 2033
The Global Bladder Cancer Therapeutics Market size is estimated to be valued at USD 3.12 billion in 2026 and is expected to reach USD 5.85 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 9.7% from 2026 to 2033.
Global Bladder Cancer Therapeutics Market Overview
The bladder cancer therapeutics market encompasses a range of treatment options targeting different disease stages and patient needs. Key products include chemotherapy agents, such as intravesical therapies for non-muscle invasive bladder cancer, and systemic chemotherapies for advanced stages. Immunotherapies, including immune checkpoint inhibitors and Bacillus Calmette–Guérin (BCG) therapy, enhance the body’s immune response to tumor cells and are critical in high-risk cases. Targeted therapies, such as FGFR inhibitors, address specific genetic mutations, while emerging gene and cell-based therapies are under clinical investigation. Supportive treatments, including pain management and anti-inflammatory drugs, improve patient quality of life and overall treatment adherence.
Key Takeaways
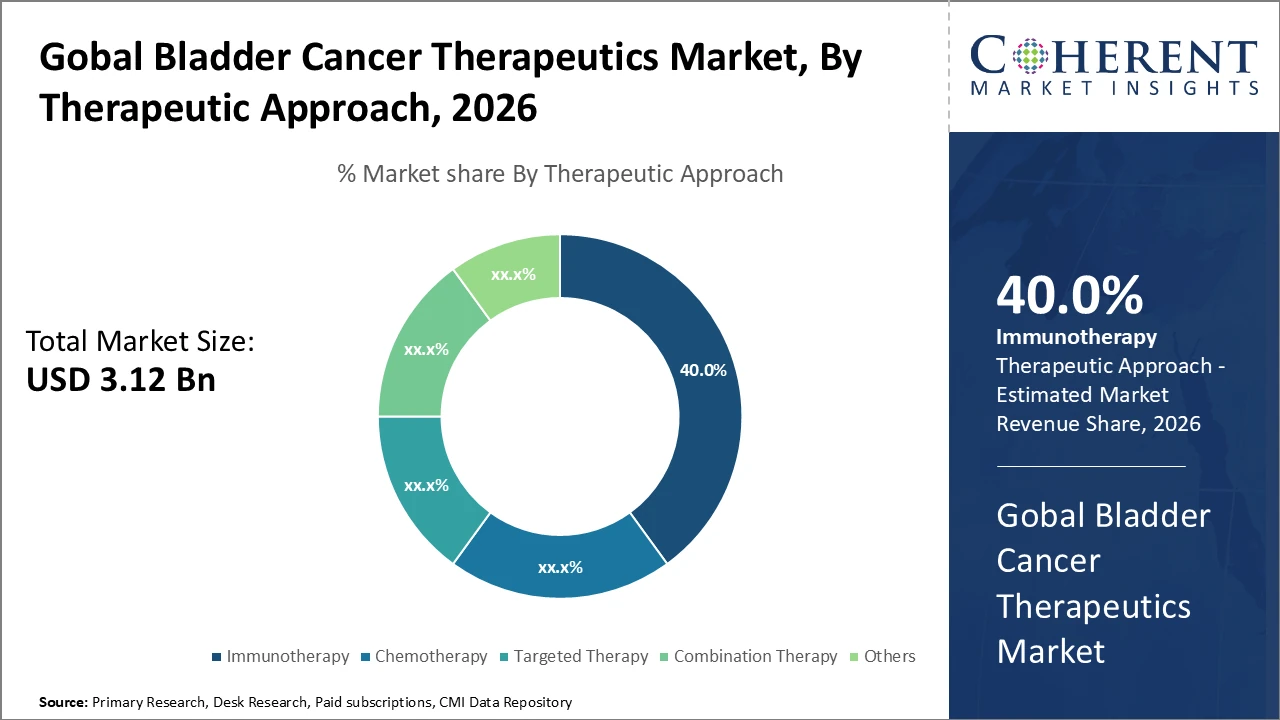
The immunotherapy subsegment leads the therapeutic approach segment with a 40% market share, driven by PD-1/PD-L1 inhibitor approvals and rapid global adoption.
Targeted therapies, especially FGFR inhibitors, are the fastest-growing subsegment, supported by increasing biomarker-driven clinical applications.
In Europe, Germany and the U.K. show strong market presence due to advanced oncology infrastructure and favorable regulatory frameworks.
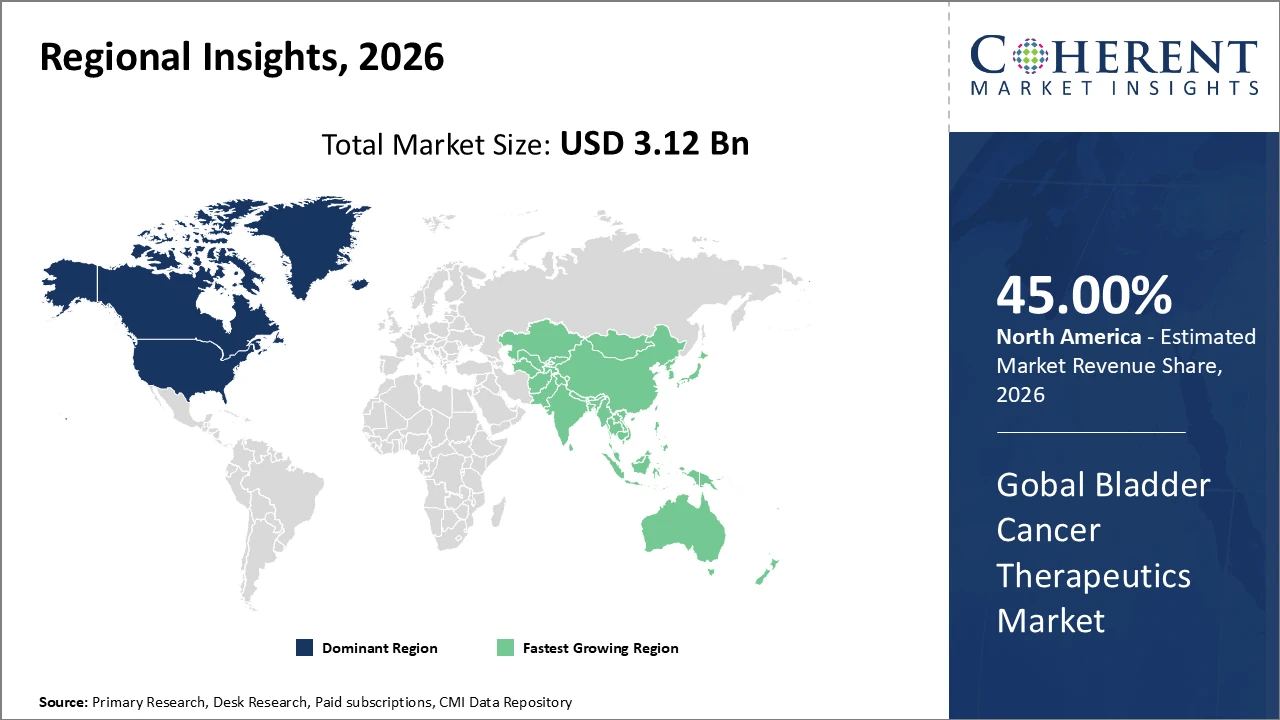
Asia Pacific, led by China and India, records the highest CAGR, fueled by rising healthcare investments and growing patient awareness.
North America remains the largest revenue contributor, supported by established reimbursement systems and high healthcare expenditure.
Bladder Cancer Therapeutics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Bladder Cancer Therapeutics Market Insights, By Therapeutic Approach
Immunotherapy leads the bladder cancer therapeutics market, driven by rapid adoption of checkpoint inhibitors and vaccine therapies that improve survival and reduce toxicity compared to conventional treatments. Its growth is reinforced by approvals of new PD-1/PD-L1 inhibitors, particularly in North America and Europe. Combination therapy is the fastest-growing segment, supported by clinical trials exploring synergistic effects of immunotherapies paired with targeted agents to overcome resistance and enhance efficacy. Chemotherapy remains foundational but is gradually declining due to side effects, while targeted therapies, including FGFR inhibitors for biomarker-positive patients, drive incremental growth. The others segment covers emerging therapies in early clinical stages.
Bladder Cancer Therapeutics Market Insights, By Drug Type
Checkpoint inhibitors dominate the bladder cancer therapeutics market, driven by proven efficacy in first- and second-line settings and widespread adoption in developed markets, generating multi-billion-dollar revenues in 2025. FGFR inhibitors represent the fastest-growing subsegment, supported by precision medicine advances and ongoing global phase III trials targeting FGFR3-mutated patients. Antibody-drug conjugates (ADCs) are gaining traction due to their targeted cytotoxic delivery, minimizing systemic side effects and showing potential in refractory cases. BCG immunotherapy remains important for non-muscle invasive bladder cancer but faces competition from newer agents. The others segment includes emerging biologics and gene therapies still in clinical investigation.
Bladder Cancer Therapeutics Market Insights, By End-User
Hospitals dominate the bladder cancer therapeutics market due to their comprehensive treatment capabilities and patient access. Their leadership is supported by infrastructure that enables administration of complex therapies and centralized management, attracting most immunotherapy and combination treatment cases. Specialty centers are the fastest-growing end-user subsegment, offering advanced diagnostics and personalized care models, driven by growing expertise and patient preference for specialized settings. Oncology clinics provide accessible outpatient services with increasing uptake of innovative therapies, while the others segment includes home healthcare and clinical research facilities adopting emerging treatment technologies.
Bladder Cancer Therapeutics Market Trends
The bladder cancer therapeutics market is increasingly shifting toward personalized medicine, driven by biomarker-guided drug development that improves treatment specificity and efficacy.
Clinical adoption of combination immunotherapies rose by 18% in 2025, reflecting evolving therapeutic protocols.
Integration of digital therapeutics and companion diagnostics is influencing treatment decisions, highlighted by the 2026 rollout of AI-powered platforms that enhance patient stratification.
Bladder Cancer Therapeutics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Bladder Cancer Therapeutics Market Analysis and Trends
In North America, the bladder cancer therapeutics market is dominated by advanced healthcare infrastructure, favorable regulatory frameworks, and the presence of leading players such as Merck and Astellas Pharma. The region holds around 45% of the global market share in 2026, driven by the extensive adoption of immunotherapies and combination treatment protocols. Well-established reimbursement systems and high patient diagnosis rates further support market growth, enabling wider access to innovative therapies. Strong research and development activities, coupled with early adoption of novel drugs, reinforce the region’s leadership position and contribute significantly to revenue generation in the global bladder cancer therapeutics market.
Asia Pacific Bladder Cancer Therapeutics Market Analysis and Trends
The Asia Pacific bladder cancer therapeutics market is projected to witness the fastest growth, with a CAGR exceeding 12% during the forecast period. This rapid expansion is fueled by increasing government initiatives aimed at improving cancer care infrastructure and enhancing patient access to advanced treatments. Rising healthcare expenditure, growing awareness about bladder cancer, and expanding research and development investments by multinational companies are driving market adoption. Countries such as China and India are leading the regional growth through local clinical trials, establishment of specialty treatment centers, and improved diagnostic capabilities, creating significant opportunities for market players to capture emerging patient populations.
Bladder Cancer Therapeutics Market Outlook for Key Countries
USA Bladder Cancer Therapeutics Market Analysis and Trends
The U.S. bladder cancer therapeutics market is characterized by strong innovation, extensive clinical trials, and high patient adoption. In 2025, the introduction of multiple FDA-approved checkpoint inhibitors drove a revenue increase of over 10%. Leading companies such as Pfizer and Bristol-Myers Squibb have strengthened their portfolios through strategic collaborations and new immune-oncology product launches, supporting sustained market leadership through 2033. The country’s advanced healthcare infrastructure, widespread diagnostic capabilities, and favorable reimbursement policies continue to facilitate rapid commercialization of therapies, ensuring high patient access and reinforcing the United States as a dominant contributor to global bladder cancer therapeutics revenue.
Germany Bladder Cancer Therapeutics Market Analysis and Trends
Germany’s bladder cancer therapeutics market serves as a key European hub, driven by strong government funding, stringent regulatory frameworks, and investments from major players such as Roche and Bayer. In 2026, the market recorded a 9% revenue increase, supported by improved diagnostic capabilities and expanded patient access programs. The country’s focus on research-driven clinical protocols and the development of specialized oncology centers creates an ecosystem conducive to innovation. These factors collectively encourage adoption of advanced therapies, support market expansion, and position Germany as a strategic growth driver in the European bladder cancer therapeutics landscape.
Analyst Opinion
The surge in immunotherapy adoption drives market revenue, as checkpoint inhibitors and vaccine-based therapies demonstrate promising clinical outcomes. In 2024, the FDA approved an additional PD-L1 inhibitor for bladder cancer, leading to a 15% increase in patient uptake in the U.S., signaling a shift toward immune-oncology agents as front-line treatment.
Production capacity expansions for next-generation therapeutics, such as antibody-drug conjugates, increased supply-side resilience. In 2025, leading manufacturers boosted output by ~25% to meet rising demand in North America and Europe, reducing lead times and stabilizing pricing.
Diversification of therapeutic pipelines with novel targets, like FGFR3 gene mutations, has expanded market segmentation. Phase III clinical trials for FGFR inhibitors attracted significant capital, reflecting confidence in broader therapy applications and supporting growth strategies.
Geographic demand variability affects import-export dynamics. Strong import activity in Asia Pacific from European biotech firms rose by 20% in 2026, highlighting inter-regional trade dependencies and prompting optimized supply chain strategies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3.12 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.7% | 2033 Value Projection: | USD 5.85 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Astellas Pharma Inc., Pfizer Inc., Merck & Co., Inc., AstraZeneca plc, Eli Lilly and Company, Bayer AG, Ipsen, Novartis AG, Amgen Inc., Immunomedics, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Bladder Cancer Therapeutics Market Growth Factors
The rising global incidence of bladder cancer, particularly among aging populations, sustains strong demand for advanced therapeutics. In 2025, the American Cancer Society reported a 5% annual increase in new U.S. cases. Advances in genomics and molecular diagnostics have enabled precision medicine approaches, fueling clinical trial pipelines targeting specific biomarkers and accelerating market growth. Rising healthcare expenditure in emerging economies improves access to costly novel therapies, supported by policy reforms in regions like Asia Pacific that enhance oncology research infrastructure. Additionally, evolving reimbursement frameworks in North America and Europe incentivize adoption of innovative bladder cancer treatments, boosting overall market revenue.
Bladder Cancer Therapeutics Market Development
In 2025, Merck expanded its bladder cancer therapeutics portfolio through the launch of a next-generation PD-1 inhibitor, targeting both first- and second-line treatment settings. This development strengthened the company’s market presence in North America and Europe, contributing to a reported 12% increase in regional revenues. The launch also accelerated adoption of immunotherapy protocols across major oncology centers, reinforcing Merck’s competitive positioning and supporting broader clinical research initiatives in combination therapies.
Key Players
Leading Companies of the Market
Astellas Pharma, Inc.
Pfizer, Inc.
Merck & Co., Inc.
AstraZeneca plc
Ely Lilly and Company
Bayer AG
Ipsen
Novartis AG
Amgen Inc.
Immunomedics, Inc.
Competitive strategies in the bladder cancer therapeutics market focus on collaborative R&D, strategic acquisitions, and licensing agreements. In 2025, Pfizer partnered with Seagen to accelerate antibody-drug conjugate development, enhancing its clinical trial pipeline and strengthening market positioning. AstraZeneca’s 2024 acquisitions expanded their FGFR inhibitor portfolio, driving market share growth in the Asia Pacific region. Similarly, Merck entered licensing agreements with regional biotech firms, bolstering local market presence and improving patient access to innovative therapies. These strategic initiatives enable companies to diversify pipelines, optimize regional penetration, and maintain competitive advantage in the rapidly evolving bladder cancer therapeutics landscape.
Bladder Cancer Therapeutics Market Future Outlook
In the bladder cancer therapeutics market, companies are leveraging collaborative R&D, strategic acquisitions, and licensing agreements to strengthen their competitive positions. In 2025, Pfizer partnered with Seagen to accelerate antibody-drug conjugate development, enhancing clinical trial pipelines and expanding market presence. AstraZeneca’s 2024 acquisitions broadened its FGFR inhibitor portfolio, supporting market share growth in the Asia Pacific region. Meanwhile, Merck entered licensing agreements with regional biotech firms, improving patient access to innovative therapies and reinforcing local market presence. These strategies enable diversification of product pipelines, optimized regional penetration, and sustained leadership in a rapidly evolving global bladder cancer therapeutics market.
Bladder Cancer Therapeutics Market Historical Analysis
The bladder cancer therapeutics market has experienced steady growth over the past decade, driven by rising incidence rates, aging populations, and advances in oncology treatments. Historically, chemotherapy remained the primary treatment, particularly for muscle-invasive and advanced-stage bladder cancer. The approval of immunotherapies, including PD-1/PD-L1 checkpoint inhibitors in the late 2010s, marked a significant shift toward targeted and immune-oncology approaches. Adoption of combination therapies and emerging targeted agents, such as FGFR inhibitors, further diversified treatment options. Early investments in biomarker-driven precision medicine and expanding clinical trial pipelines laid the foundation for rapid market expansion, particularly in North America and Europe.
Sources
Primary Research Interviews:
Oncologists
Urologists
Oncology Pharmacists
Clinical Trial Investigators
Databases:
American Cancer Society (ACS) Reports
Global Cancer Observatory (GLOBOCAN)
SEER Cancer Statistics
WHO Cancer Statistics
IQVIA Health Data
Magazines:
Oncology Times
Cancer Therapy Advisor
PharmaTimes
BioPharma Reporter
Targeted Oncology
Journals:
Journal of Clinical Oncology
Cancer Research
Clinical Cancer Research
Urologic Oncology: Seminars and Original Investigations
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
American Cancer Society (ACS)
International Bladder Cancer Group (IBCG)
National Cancer Institute (NCI)
Bladder Cancer Advocacy Network (BCAN)
European Association of Urology (EAU)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients