Bispecific Antibodies Market Size and Forecast – 2026 – 2033
The Global Bispecific Antibodies Market size is estimated to be valued at USD 4.2 billion in 2025 and is expected to reach USD 12.7 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 16.4% from 2025 to 2032.
Global Bispecific Antibodies Market Overview
Bispecific antibody products are advanced biologic therapeutics engineered to bind two different antigens simultaneously. These drugs are designed to redirect immune cells toward disease targets, particularly in oncology and autoimmune disorders. Bispecific antibodies can enhance immune activation, improve targeting precision, and reduce off-target effects. The products are typically administered via injection or infusion and require specialized manufacturing processes. Stability, specificity, and therapeutic efficacy define product performance.
Key Takeaways
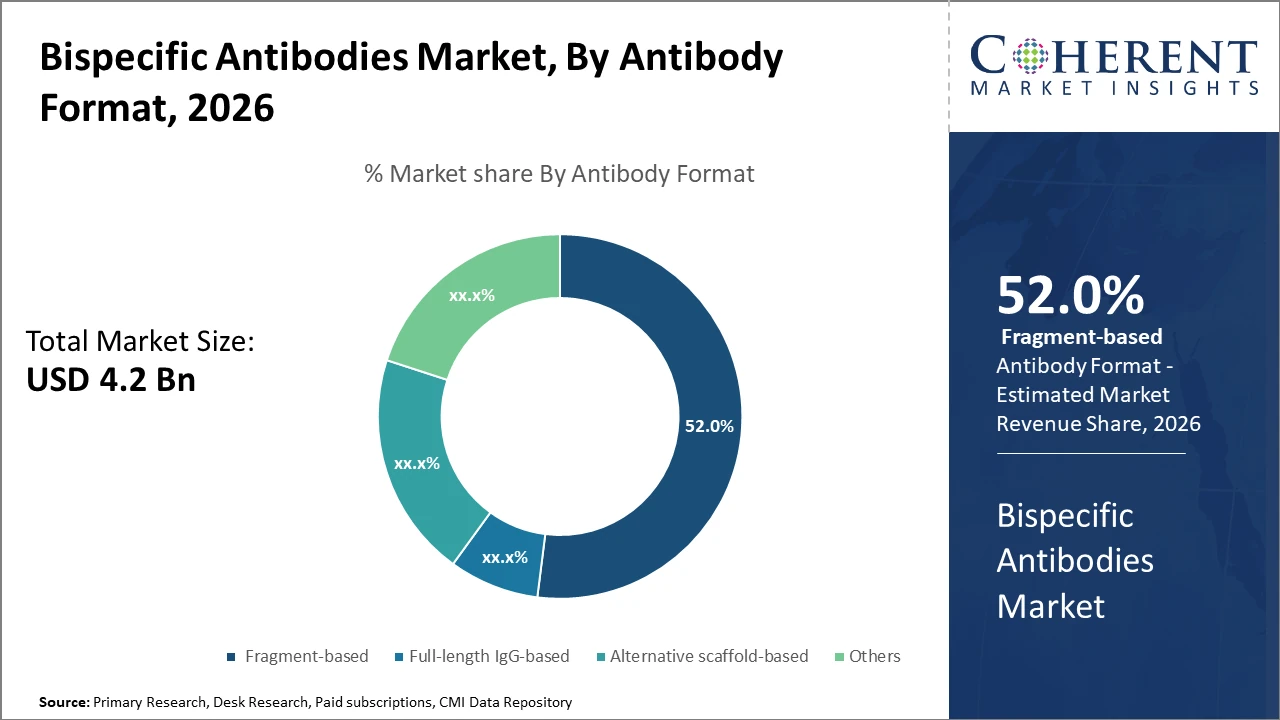
The fragment-based antibody format is driving the largest market share, attributed to its superior tumor penetration and manufacturing adaptability, with data from 2025 confirming its dominance by over 50%.
Oncology remains the largest therapeutic application, accounting for nearly 60% of overall market revenue, supported by rising clinical approvals and utilization in hematologic malignancies.
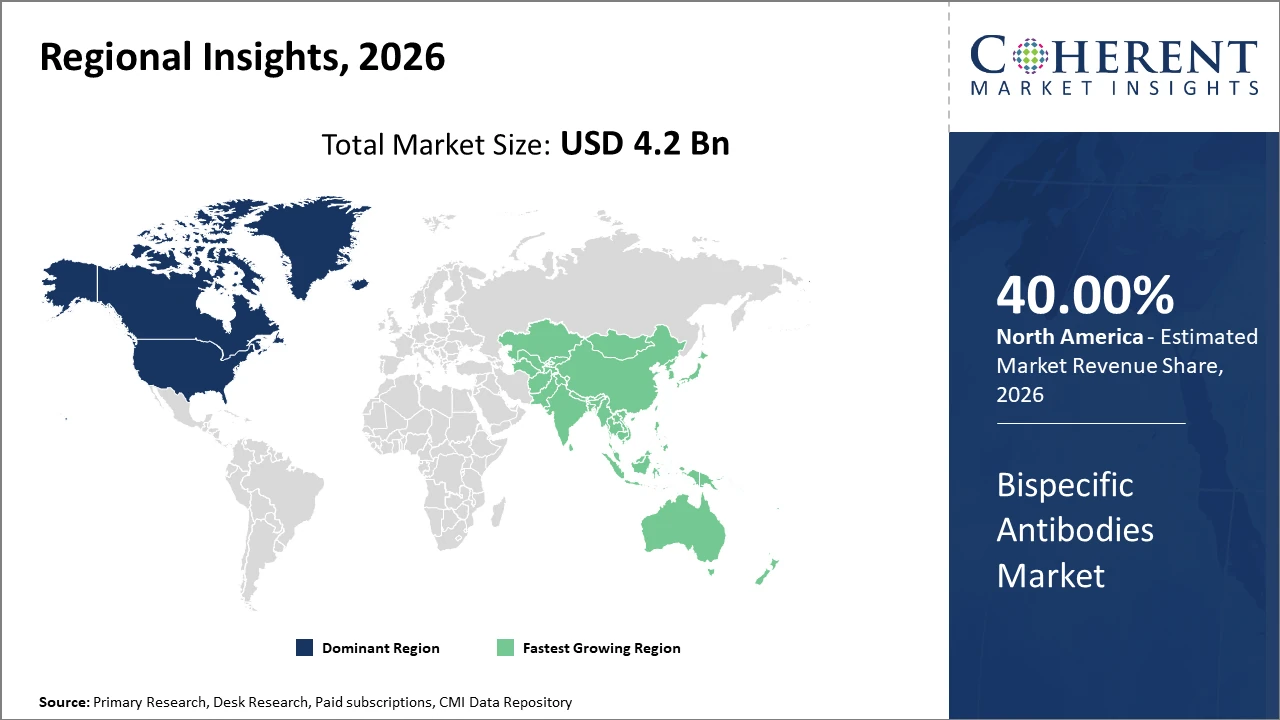
North America holds the dominant regional share, exceeding 40% of the market revenue in 2025, fueled by strong pharmaceutical infrastructure and government bioscience initiatives.
Asia Pacific is the fastest-growing region with a CAGR over 20%, driven by increasing biopharma investments, improving healthcare infrastructure, and expanding patient population, particularly in China and India.
Bispecific Antibodies Market Segmentation Analysis
To learn more about this report, Download Free Sample
Bispecific Antibodies Market Insights, By Antibody Format
Fragment-based antibodies dominate the market share at 52%. Fragment-based bispecific antibodies are favored for their smaller molecular size, which facilitates superior tumor tissue penetration and better pharmacokinetics, making them especially effective in solid tumors. The fastest-growing subsegment is Alternative scaffold-based antibodies, which offer versatile binding frameworks beyond traditional antibody structures, allowing targeting of unconventional epitopes.
Bispecific Antibodies Market Insights, By Therapeutic Application
Oncology dominates the market share at nearly 60%. Oncology drives the market due to a growing number of cancer indications addressed by bispecific formats, such as hematologic malignancies (e.g., multiple myeloma) and hard-to-treat solid tumors. Advances in T-cell engager bispecific antibodies and immune checkpoint inhibitor combinations have greatly enhanced clinical outcomes, evident from a 20% rise in oncology-related bispecific antibody revenue in 2024. Autoimmune Diseases represent the fastest-growing subsegment, propelled by increasing recognition of bispecific antibodies’ ability to modulate immune responses precisely. Conditions like rheumatoid arthritis and psoriasis have seen early clinical successes, validated by multiple phase II/III trials, boosting market interest post-2023.
Bispecific Antibodies Market Insights, By End-User
Hospitals dominate the market share due to being primary administration points for complex therapeutic interventions, including bispecific antibody infusions. Hospitals’ extensive oncology wards and infrastructure facilitate large patient volumes and continuous therapy, driving consistent market revenue. Specialty Clinics are the fastest-growing subsegment as their proliferation enables focused treatment, such as autoimmune and rare disease management, where bispecific antibodies play an increasing role. These clinics benefit from personalized patient care models that maximize therapeutic efficacy and patient adherence. Research Institutes contribute by advancing clinical trials and novel antibody discovery, forming the innovation backbone of the market.
Bispecific Antibodies Market Trends
The market is witnessing transformative trends that redefine therapeutic possibilities. One significant trend is the rise of trispecific and multi-targeted antibodies, which have recently shown improved clinical outcomes by engaging multiple antigens simultaneously.
For example, in 2025, a trispecific antibody candidate demonstrated enhanced survival rates in phase II trials for aggressive lymphoma, indicating progression beyond conventional bispecific designs.
Moreover, artificial intelligence-driven antibody engineering is revolutionizing drug discovery, with accelerated design cycles reducing lead times by up to 30%, as observed in 2024 biotech initiatives.
Another trend is the increasing penetration of biosimilar bispecifics to address cost limitations in emerging markets, with several candidates in late-stage development aiming to capture growing demand in the Asia Pacific and Latin America.
Bispecific Antibodies Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Bispecific Antibodies Market Analysis and Trends
In North America, the Bispecific Antibodies market dominance stems from strong pharmaceutical research hubs, substantial biotech presence, and government incentives fostering innovation and clinical trials. The region commanded over 40% market share in 2025, driven by companies like Amgen and Genentech, who maintain aggressive pipelines and substantial manufacturing capabilities. Regulatory frameworks favoring fast-track approvals have accelerated product launches and elevated market revenue growth.
Asia Pacific Bispecific Antibodies Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, registering a CAGR exceeding 20% through 2032. This surge is propelled by increasing healthcare expenditure, expanding patient base, and rising adoption of biologics in countries like China and India. Government efforts to enhance biopharma infrastructure and local production partnerships with global market players contribute to this rapid expansion.
Bispecific Antibodies Market Outlook for Key Countries
USA Bispecific Antibodies Market Analysis and Trends
The USA’s market is the largest contributor to overall revenue, supported by an extremely large oncology patient pool and an advanced clinical research framework. Notably, the FDA’s Oncology Center of Excellence expedited over 15 bispecific antibody approvals between 2023 and 2025, affirming the market’s maturity. Leading companies such as Regeneron Pharmaceuticals and Pfizer have leveraged strategic acquisitions and licensing deals to strengthen their U.S. market footprint, facilitating continuous product innovation and market revenue growth.
China Bispecific Antibodies Market Analysis and Trends
China's market is rapidly expanding, driven primarily by governmental support for biotech innovation and increasing investment in antibody therapeutics manufacturing. In 2024, collaborations between local biotech firms and multinational corporations accelerated the development and commercialization of bispecific antibodies tailored for prevalent cancers like lung and liver cancer. The establishment of specialized antibody manufacturing parks and regulatory reforms have augmented market growth, positioning China as a critical regional hub.
Analyst Opinion
The expanding pipeline of bispecific antibody molecules indicates a robust supply-side growth. For instance, over 150 bispecific antibody candidates were in various clinical trial phases in 2024, reflecting intensified production capacity and R&D investment. This has directly contributed to a 12% increase in market revenue from clinical-stage assets year-over-year in 2024.
On the demand side, the rising prevalence of hematological malignancies and solid tumors is fueling market growth. Data from 2025 reveals that new cancer cases treated with bispecific antibodies grew by nearly 18% globally. This increase reflects extensive adoption across oncology, validating the market’s strong growth trajectory.
Micro-level indicators show an upsurge in personalized medicine approaches, with bispecific antibodies uniquely positioned to address complex disease pathways. In 2024, precision oncology programs incorporating bispecific antibodies reported patient response rates improving by 25% compared to traditional monoclonal antibodies, boosting demand in high-income countries.
Nano-scale production techniques, reducing manufacturing costs while enhancing antibody purity, have driven market expansion. Recent studies in 2025 demonstrated that innovative cell culture bioprocessing methods decreased production costs by 15%, allowing emerging market companies to enter the market, thereby broadening the market scope globally.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 4.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 16.4% | 2033 Value Projection: | USD 12.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Merck & Co., Bristol-Myers Squibb, MacroGenics Inc., Immunocore Limited, IGM Biosciences, Xencor Inc., Zymeworks Inc., F. Hoffmann-La Roche Ltd, Daiichi Sankyo, Novartis AG. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Bispecific Antibodies Market Growth Factors
The growing burden of cancer globally acts as a critical market driver, with estimates indicating over 20 million new cancer cases annually influencing product demand. Technological advancements in antibody engineering have facilitated the creation of highly effective bispecific formats, improving therapeutic efficacy and market adoption. Expanding government support and fast-track approvals across major pharmaceutical hubs have reduced regulatory barriers, allowing quick commercialization of novel bispecific antibodies. Furthermore, increasing investments in contract manufacturing organizations (CMOs) specializing in biologics have enhanced production scalability, fueling business growth and market expansion.
Bispecific Antibodies Market Development
In June 2025, BioNTech and Bristol Myers Squibb (BMS) entered into a strategic co-development agreement for BioNTech’s BNT327, a next-generation bispecific antibody targeting solid tumors. The collaboration aims to accelerate clinical development and expand treatment options in oncology by leveraging complementary expertise in immuno-oncology research and global commercialization.
In December 2024, Bizengri (zenocutuzumab) received regulatory approval for the treatment of NRG1 fusion–positive lung and pancreatic cancers, marking a significant advancement for patients with rare oncogenic drivers. This approval strengthened the targeted oncology therapeutics landscape by validating precision medicine approaches for difficult-to-treat solid tumors.
Key Players
Leading Companies of the Market
Merck & Co.
Bristol-Myers Squibb
MacroGenics Inc.
Immunocore Limited
IGM Biosciences
Xencor Inc.
Zymeworks Inc.
F. Hoffmann-La Roche Ltd
Daiichi Sankyo
Novartis AG
Several market companies have adopted strategic collaborations and licensing agreements to accelerate product pipeline development. For example, a leading company’s partnership with biotech firms in 2024 resulted in a 30% reduction in drug-to-market time. Additionally, investments in next-generation bispecific platforms enabled other entities to expand their market share by capitalizing on unmet clinical needs, achieving considerable revenue growth and competitive advantage.
Bispecific Antibodies Market Future Outlook
The future outlook remains highly favorable, supported by expanding oncology indications and growing interest in autoimmune and inflammatory diseases. Advances in protein engineering and bioprocessing are expected to improve scalability and reduce costs. Combination therapies and personalized medicine approaches will further broaden applications. Regulatory familiarity with bispecific formats will streamline approvals. The market is likely to become a major pillar of next-generation biologics.
Bispecific Antibodies Market Historical Analysis
The bispecific antibodies market evolved as pharmaceutical companies sought to improve therapeutic efficacy beyond conventional monoclonal antibodies. Early development faced significant challenges related to protein stability, manufacturing complexity, and immune safety. Initial clinical successes in oncology validated the therapeutic concept, leading to regulatory approvals that transformed market confidence. Pharmaceutical companies rapidly expanded research pipelines, focusing on novel antibody formats and production methods. Strategic collaborations and licensing agreements accelerated development and commercialization efforts.
Sources
Primary Research Interviews:
Oncologists
Immunologists
Biologics researchers
Pharmaceutical executives
Databases:
FDA Biologics Database
ClinicalTrials.gov
Statista Biopharmaceutical Data
EMA Drug Database
Pharmaprojects
Magazines:
Nature Biotechnology
FierceBiotech
BioPharma Dive
Genetic Engineering & Biotechnology News
Pharmaceutical Technology
Journals:
Nature Reviews Drug Discovery
Journal of Clinical Oncology
Cancer Research
Clinical Cancer Research
The Lancet Oncology
Newspapers:
Reuters Pharma
Financial Times (Healthcare)
The Wall Street Journal (Life Sciences)
Bloomberg Health
The Guardian (Science)
Associations:
Biotechnology Innovation Organization
American Association for Cancer Research
European Society for Medical Oncology
International Society for Biological Therapy of Cancer
PhRMA
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients