Biotechnology Market is estimated to be valued at USD 1,034.63 Bn in 2025 and is expected to reach USD 2,330.47 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 12.3% from 2025 to 2032.
Analysts’ views on Global Biotechnology Market:
Increasing prevalence of chronic diseases, new product launches, and strategies like mergers, acquisitions, and collaboration are expected to drive the global biotechnology market growth over the forecast period. For instance, according to the data published by the World Health Organization, on February 3, 2022, cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. Diabetes caused nearly 2.0 million while cardiovascular and respiratory diseases caused nearly 17.9 and 4.1 million deaths in 2020, respectively, worldwide. The increasing prevalence of these diseases increases the need to carry out diagnostic tests and treatment using biotechnological techniques which ultimately boosts demand for biotechnology.
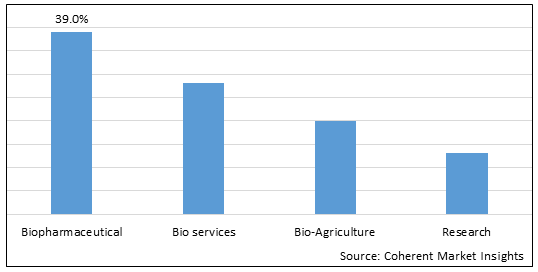
Figure 1. Global Biotechnology Market Share (%), By Application, 2025
To learn more about this report, Download Free Sample
Global Biotechnology Market – Drivers
Increasing research and development activities in tissue culture and cell engineering
Increasing research and development in the field of cell and tissue engineering is expected to drive the global biotechnology market growth over the forecast period. For instance, on August 16, 2022, Bharat Biotech., an India-based vaccines & bio-therapeutics manufacturer, announced phase III clinical trial data for its intranasal COVID-19 vaccine. The vaccine was proved to be safe well tolerated and immunogenic in subjects in controlled clinical trials. Drug Controller Genaral of India (DCGI) has also granted permission to the firm to conduct a phase-3 clinical trial to compare the immunogenicity and safety of BBV-154 (intranasal) with Covaxin. This trial has been permitted to be conducted at nine sites in India.
Increasing launches of new products in biotechnology
Increasing launch of new products in biotechnology is expected to drive the global biotechnology market growth. For instance, on July 4, 2023, Niocon, India based biopharmaceutical company, launched a biosimilar version of AbbVie's top-selling biologic Humira (generically called adalimumab) in the U.S. market under the brand name HULIO. Adalimumab is used to treat certain inflammatory diseases like rheumatoid arthritis. The drug has been made available to patients in the United States after five years of experience in Europe and two years in Canada.
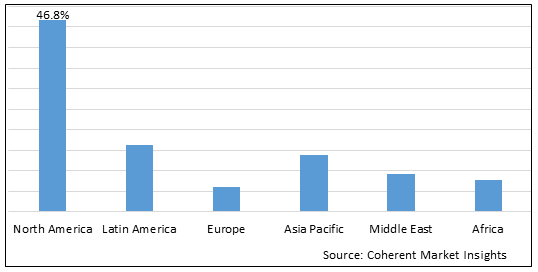
Figure 2. Global Biotechnology Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Biotechnology Market - Regional Analysis
Among region, North America is estimated to hold a dominant position in the global biotechnology market over the forecast period, owing to increasing launches of products. For instance, on February 11, 2025, Eli Lilly and Company, a U.S.-based pharmaceutical company, received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for bebtelovimab, an antibody that demonstrates neutralization against the Omicron variant. Bebtelovimab is used for the treatment of mild to moderate COVID-19 in adults and pediatric patients. The authorized dose of bebtelovimab is 175 mg given as an intravenous injection over at least 30 seconds.
Global Biotechnology Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global biotechnology market, due to increased demand for biotechnological products and vaccines for treatment. For instance, on November 9, 2020, Pfizer Inc., U.S. based multinational pharmaceutical and biotechnology corporation, announced a vaccine candidate against COVID-19. It is an mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 It has demonstrated evidence of efficacy against COVID-19 in participants without prior evidence of SARS-CoV-2 infection, based on the first interim efficacy analysis conducted by external independent data monitoring committee. The protection against infection is achieved after 28 days of initiation of vaccination.
Biotechnology Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,034.63 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.3% | 2032 Value Projection: | USD 2,330.47 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Amgen Inc., GlaxoSmithKline, Johnson and Johnson, Merck, Novartis, Novo Nordisk, Pfizer, Inc., Roche, and Sanofi – Aventis. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Biotechnology Market Segmentation:
Global biotechnology market report is segmented into application, technology, and region
Based on Application, the global biotechnology market is segmented into biopharmaceutical, bio services, bio-agriculture, and research. Out of which, the biopharmaceutical segment is expected to dominate the market due to the launch of newer products.
Based on Technology, the global biotechnology market is segmented into fermentation, tissue engineering, PCR technology, nanobiotechnology, chromatography, DNA sequencing technology, cell-based assay, and other technologies. Among these, the DNA sequencing technology segment is expected to dominate the market over the forecast period due to increasing research and development of DNA sequencing technology in various applications.
Based on Region, the global biotechnology market is segmented into North America, Latin America, Europe, Asia Pacific, and Middle East & Africa. Among these, North America segment is expected to dominate the market due to the increasing launch of new products.
Among all segmentation, the application segment has the highest potential due to the increasing launches of products by the key market players. For instance, on March 14, 2023, Eli Lilly and Company, U.S. based pharmaceutical company, entered into the dermatological space with the launch of Copellor (Ixekizumab), a product to treat moderate-to-severe plaque psoriasis. The drug is designed to specifically target a protein that plays a role in triggering and maintaining inflammation in psoriasis.
Global Biotechnology Market Cross Sectional Analysis:
Introduction of newer products and technologies in biotechnology by key market players in Europe region is expected to drive the growth of the application segment in the region. For instance, Illumina, Inc., U.S. based biotechnology company, launched a new in vitro diagnostic test in Europe designed to profile various cancer mutations and help direct patients to targeted therapies. The new TruSight Oncology Comprehensive test kit can examine multiple cancer genes and biomarkers. Those include 517 cancer-relevant genes in DNA and RNA spanning nearly 30 solid tumor types, combined with other genomic signatures such as microsatellite instability and tumor mutational burden. According to Illumina, the one test eliminates the need for clinicians to run sequential gene assays on multiple tissue biopsy samples.
Global Biotechnology Market: Key Developments
On June 29, 2022, Bayer AG, a Germany-based German multinational pharmaceutical and biotechnology company, opened a new U.S. cancer research and innovation center to the tune of US$ 140 million. It is a 62,100-square-foot precision molecular oncology research center, home to about 1000 biotechs.
On May 13, 2022, F. Hoffmann-La Roche Ltd, a Switzerland-based healthcare company, announced that it has made the breast cancer antibody cocktail drug PHESGO available in India, which will be priced 20% less and will be more convenient to administer than existing therapy. PHESGO is a fixed dose combination of two monoclonal antibodies Perjeta (pertuzumab) and Herceptin (trastuzumab) in Oncology for the treatment of HER - 2 positive breast cancer. The drug is approved for treating both early and late-stage or metastatic HER2-positive breast cancer.
On May 23, 2023, ReNAgade Therapeutics, U.S. based company manufacturing RNA medicines to correct disease, announced a US$ 300 million Series A financing round led by MPM BioImpact, U.S. based biotechnology investment firm, and F2 Ventures, Israel based healthcare investment platform. ReNAgade has built a comprehensive and complementary platform that combines its proprietary delivery technologies, including novel lipid nanoparticles (LNPs), with a broad array of coding, editing, and gene insertion tools, in an all-RNA system. ReNAgade aims to address major limitations in RNA therapeutics by enabling the delivery of RNA medicines to previously inaccessible tissues and cells in the body, substantially expanding the potential addressable disease market.
On May 25, 2023, Pfizer Inc., announced U.S. FDA approval of PAXLOVID (nirmatrelvir tablets and ritonavir tablets) for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. The data showed an 86% reduction in risk of COVID-19-related hospitalization or death from any cause through Day 28 in patients who initiated treatment with PAXLOVID within five days of symptoms onset, compared to placebo. The approval was further supported by results of the secondary endpoint of phase 2/3 Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients study, which showed a numerical reduction in COVID-19-related hospitalizations or death from any cause through Day 28 in a sub-group of non-hospitalized adults with confirmed COVID 19 infection.
Global Biotechnology Market: Key Trends
Introduction of newer research and development activities
Introduction of newer and more efficient research and development activities to boost the biotechnological field can drive the growth of the market. On August 12, 2022, Mount Sinai Health System, U.S. based hospital network, and Icahn School of Medicine at Mount Sinai, U.S. based research-based medical school, launched a new human genome sequencing research project called the Mount Sinai Million Health Discoveries Program with the Regeneron Genetics Center (RGC), part of U.S. based biotechnology company Regeneron. The Program aims to enroll one million Mount Sinai patients over five years. Its goal is to provide researchers with a unique data set that will help them assess the true potential of genetics-based, precision medicine approaches to guide everyday patient care, as well as to generate new insights to guide the discovery and development of potential new therapies. With this collaboration, researchers plan to combine RGC’S gene sequencing capabilities and scientific research expertise with Mount Sinai’s diverse patient population and advanced electronic health records systems.
Acquisition strategy by key market players
Acquisition strategy by key market players is expected to drive growth of global biotechnology market in forecast period. For instance, on December 8, 2021, Thermo Fisher Scientific Inc. U.S. based supplier of scientific instrumentation, reagents and consumables, and software services, announced it has completed the acquisition of PPD, Inc., U.S. based global provider of clinical research services to the biopharma and biotech industry for US$ 17.4 billion. With the addition of PPD, Thermo Fisher can offer services across the clinical development spectrum − from scientific discovery to assessing safety, efficacy, and health care outcomes, to managing clinical trial logistics, to the development and manufacturing of the drug product.
Global Biotechnology Market: Restraints
Termination of clinical trials
The failure in obtaining expected results causes the termination of the clinical trial. Such failures can hamper the global biotechnology market growth. For instance, on July 7, 2022, Legend Biotech Corporation, U.S. based commercial-stage biotechnology company developing and manufacturing novel therapies, notified the U.S. Food and Drug Administration (FDA) that Legend Biotech has terminated its Phase 1 Clinical Trial under the Investigational New Drug (IND) application for LB1901, Legend Biotech's investigational autologous chimeric antigen receptor T-cell (CAR-T) therapy for the treatment of adults with relapsed or refractory T-cell lymphoma. The termination of the trial was based on a lack of clinical benefit from a similar Legend Biotech CAR-T product candidate expressing the same CAR protein as LB1901 which was the subject of an investigator-initiated study conducted in China.
To counterbalance this restraint, more research and development should be done that can avoid costly failures of clinical trials.
High cost of biotechnology therapies
In emerging economies, the lack of infrastructure facilities and the high cost of biotechnology therapies is expected to hamper the global biotechnology market growth. For instance, according to an article published in the Journal of managed care & Specialty Pharmacy in May 2021, average costs over a lifetime of treatment with antibodies for patients with factor VIII (FVIII) hemophilia A be between US$15 million and US$100 million, which is more than 3 times (and up to 20 times) the projected lifetime cost for a patient treated with alternative therapy.
To counterbalance this restrain, more funding by governments for reimbursement should be allocated.
Global Biotechnology Market - Key Players
Major players operating in the global biotechnology market include Abbott Laboratories, Amgen Inc., GlaxoSmithKline, Johnson and Johnson, Merck, Novartis, Novo Nordisk, Pfizer, Inc., Roche, and Sanofi – Aventis.
*Definition: Biotechnology involves the use of organisms, processes, or systems to produce products such as pharmaceuticals on a large scale. The rise in demand for these therapeutics and diagnostic solutions is expected to assist the growth of the market during the forecast period. Furthermore, increasing research and development for the production of vaccines is also expected to propel the market growth.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients