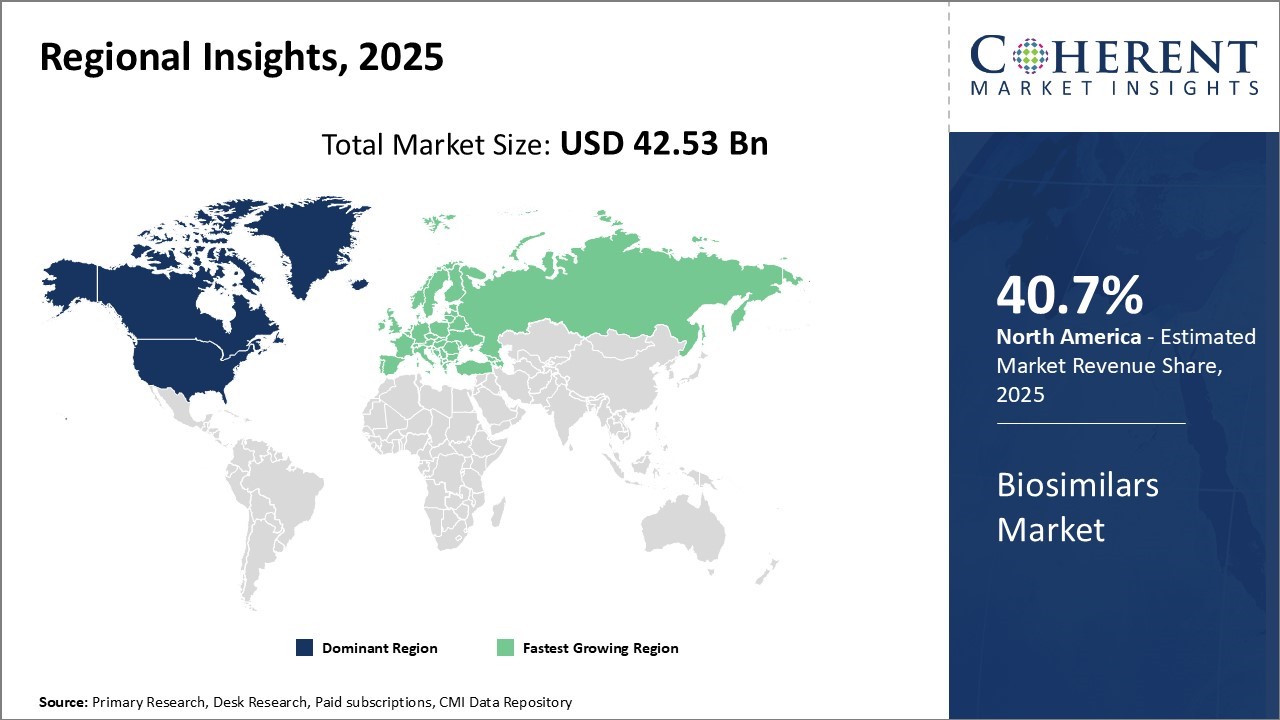
The biosimilars market is estimated to be valued at USD 42.53 Bn in 2025 and is expected to reach USD 136.37 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 18.1% from 2025 to 2032.
To learn more about this report, Download Free Sample
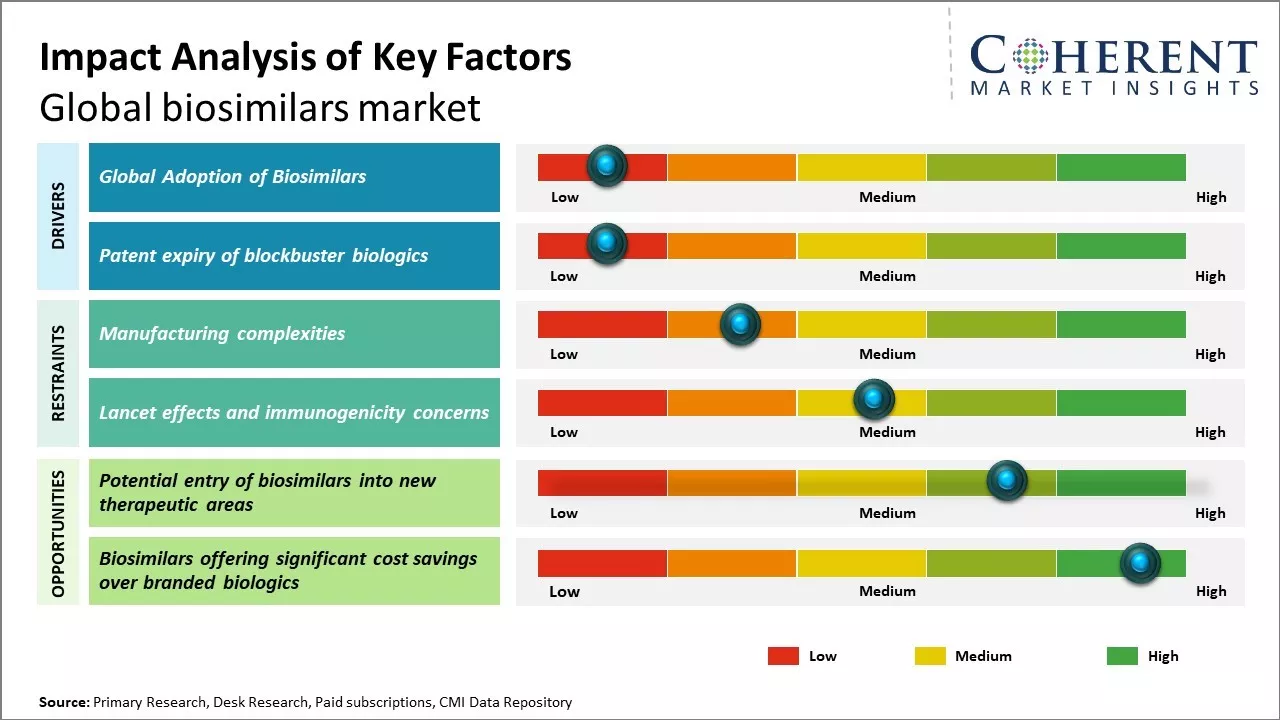
Growing demand for affordable biological drugs along with patent expiries of major biologics drugs can drive the market growth. Increasing investments by market players for research & development and production of biosimilars coupled with supportive regulatory framework can also boost demand for biosimilars. Moreover, biosimilars provide similar therapeutic efficacy in comparison to reference biologics with significant cost savings, which makes them an attractive alternative.
However, manufacturing challenges associated with biosimilars can hinder the market growth over the forecast period.
To learn more about this report, Download Free Sample
|
Current Events |
Description and its impact |
|
Legislative & Economic Pressures |
|
|
Regional Regulatory Fragmentation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of drug class, recombinant human growth hormone segment is estimated to contribute the highest market share of 20.5% in 2025, owing to its versatility and wide range of applications.
Recombinant human growth hormone is used to treat a variety of conditions related to growth hormone deficiency in both pediatric and adult patients. Some of the major conditions treated with recombinant human growth hormone include turner syndrome, Prader-Willi syndrome, chronic renal insufficiency, and AIDS-related wasting. Its ability to stimulate growth in both children and adults through different growth-promoting effects makes it a favorable treatment option for doctors and patients.
In terms of therapy type, oncology segment is estimated to contribute the highest market share of 25.12 % in 2025, owing to the high prevalence of cancer and efficacy of biosimilars in oncological treatment.
The continually growing cancer patient pool boosts demand for cost-effective oncology biosimilars. Many blockbusters biologic drugs have lost exclusivity in recent years, providing opportunities for oncology biosimilars. Areas such as non-Hodgkin's lymphoma, breast cancer, and colorectal cancer with large biologic drug consuming patient bases are prime market targets. Biosimilars of widely prescribed biologics like bevacizumab, trastuzumab, rituximab and cetuximab witness huge uptake due to demonstrated similar clinical outcomes.
In terms of distribution channel, hospital pharmacies segment is estimated to contribute the highest market share of 30.5% in 2025, owing to the potential for cost savings and bulk procurement advantages.
Hospital pharmacies treat a large volume of patients on a daily basis requiring immense drug budgets. Biosimilars enable substantial drug expenditure reductions for cash strapped hospitals. Their lower prices compared to reference biologics result in significant budgetary relief without compromising treatment quality. Furthermore, hospitals can leverage their bulk purchasing power to negotiate additional discounts on biosimilar prices from manufacturers.
Artificial Intelligence (AI) is playing an instrumental role in transforming this sector by enhancing drug development processes, optimizing manufacturing, and improving market strategies. The integration of AI within the biosimilars industry is not only accelerating time-to-market but also ensuring higher precision and cost-efficiency.
To learn more about this report, Download Free Sample
North America currently dominates the global biosimilars market, accounting for an estimated 40.7% of the global market share in 2025. With biosimilars expected to drive down healthcare costs in the U.S., the U.S. Food and Drug Administration (FDA) has approved over 20 biosimilars to date in an effort to increase competition. Major players in the U.S. market include Pfizer, Novartis, and Merck who are well positioned to leverage their pharmaceutical expertise and capture market share as familiar biosimilars continue to go off-patent.
For instance, April 2025, the U.S. Food and Drugs Administration (FDA) approved 10 biosimilars, including: Celltrion’s Avtozma, Osenvelt and Stoboclo, and Omlyclo; Samsung Bioepis’ Ospomyv and Xbryk; Sanofi-Aventis’ Merilog; Fresenius Kabi’s Bomyntra and Conexxence; and Biocon’s Jobevne.
Europe is expected to be the fastest-growing region for the market over the forecast period. Countries like Germany, France, and the U.K. have particularly strong biosimilar markets and industry presence. Germany in particular has emerged as a biosimilars manufacturing powerhouse with several local companies, attracting global biosimilar developers through its strategically strong position in the value chain.
For instance, in April 2025, the European Commission (EC) approved the denosumab (Xgeva) biosimilar Denbrayce for the prevention of skeletal-related events in adult patients with advanced malignancies involving the bone and in adult and skeletally mature adolescent patients with giant cell tumor of the bone.
In the U.S., biosimilars are gaining traction as health systems and insurers push for lower-cost alternatives to expensive biologic therapies. Intermediaries such as pharmacy benefit managers and insurer plans exert strong influence over formulary placement, driving adoption when incentives align. Regulatory clarity on interchangeable designation has begun to boost prescriber confidence, though uptake remains influenced by pricing strategies and rebate structures.
Germany stands out in Europe for its comprehensive approach to encouraging biosimilar use. Regional physician associations set annual prescribing targets backed by financial incentives or penalties, and doctors who exceed quotas may share savings with insurers. Meanwhile, legislation empowers pharmacists to substitute interchangeable biosimilars unless explicitly prohibited—reinforcing uptake through streamlined policies and proactive education among clinicians.
India’s biosimilars sector is driven by cost-sensitive health systems and global manufacturing capacity. With numerous domestic producers and high-quality production standards, Indian companies have become major exporters while maintaining affordable pricing for domestic use. Regulatory evolution and growing emphasis on biologic access support continued expansion both at home and in global markets.
China is a major driver in the biosimilars market due to its rapidly expanding biopharmaceutical sector, supportive government policies, and increasing demand for affordable biologic therapies. The Chinese government has implemented regulatory reforms to accelerate biosimilar approvals, including guidelines that align more closely with international standards.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 42.53 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 18.1% | 2032 Value Projection: | USD 136.37 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
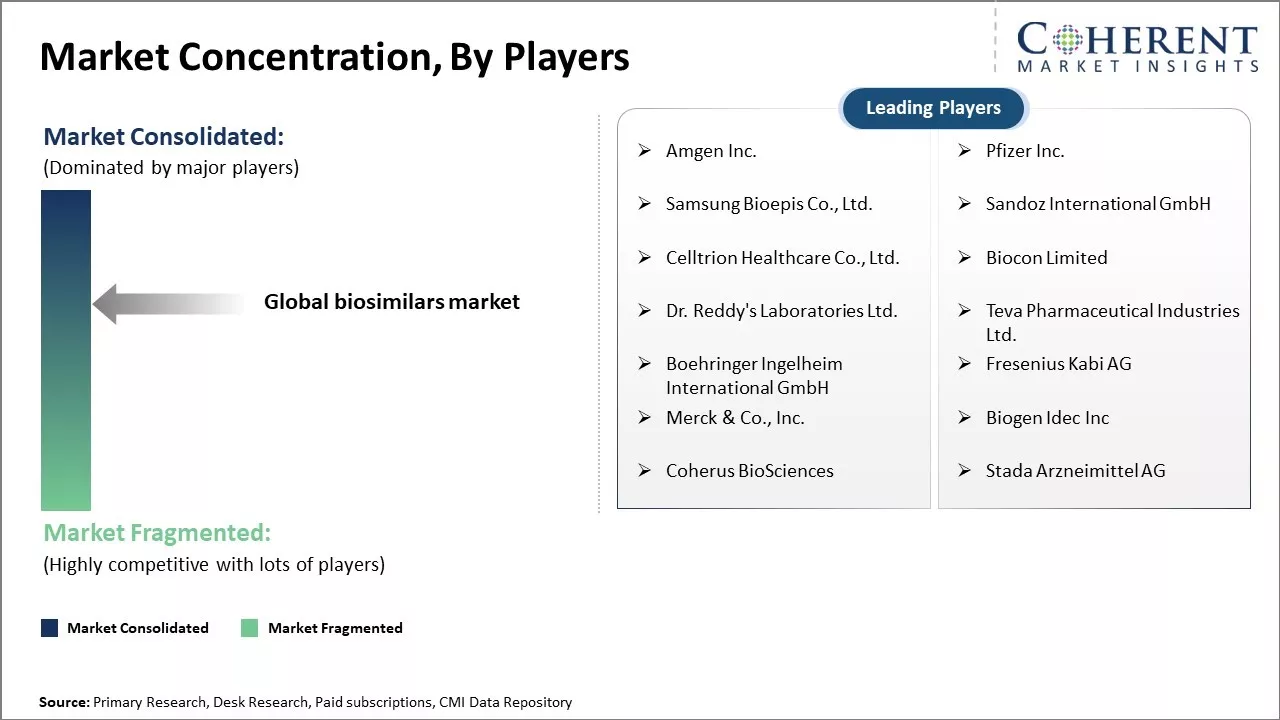
Amgen Inc., Pfizer Inc., Samsung Bioepis Co., Ltd., Sandoz International GmbH, Celltrion Healthcare Co., Ltd., Biocon Limited, Dr. Reddy's Laboratories Ltd., Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim International GmbH, Fresenius Kabi AG, Merck & Co., Inc., Biogen Idec Inc, Coherus BioSciences, and Stada Arzneimittel AG |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The global biosimilars market refers to the market for biological medicinal products that are similar to already approved original branded biologic medicines. Biosimilar medicines compete with innovator biologic medicines after the patent and exclusivity period ends. The global biosimilars market has seen significant growth in recent years as patents of large biologics expire and more biosimilars are approved. Various factors such as rising healthcare costs, patent expiries of major biologics, and regulatory support for biosimilars are driving the demand in this market across regions.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients