Biopharmaceutical Contract Manufacturing Market Size and Forecast – 2025 – 2032
The Biopharmaceutical Contract Manufacturing Market size is estimated to be valued at USD 32.5 billion in 2025 and is expected to reach USD 64.7 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 10.3% from 2025 to 2032.
Global Biopharmaceutical Contract Manufacturing Market Overview
Contract manufacturing organizations (CMOs) provide wide range of manufacturing services, which include contract packaging, quality testing, and development service to pharmaceutical and biotechnology industries. Biopharmaceutical companies prefer CMOs due to complexity in manufacturing process of biomolecules, as they consist of different shape, size, and behavior with significantly complex process than pharmaceutical drugs. Contract manufacturing organizations provide services from development of biologics to commercial scale production. Few companies also provide cell line development, fermentation, process optimization, and analytical characterization. Improved efficiency, weak product pipeline, and increasing price pressure is expected to favor the growth of biopharmaceutical contract manufacturing market in near future.
Key Takeaways
The drug substance manufacturing segment leads the biopharmaceutical contract manufacturing market share, driven by its critical role in biologics production and capacity expansions.
Mammalian cell culture dominates technology adoption due to compatibility with complex therapeutic proteins and monoclonal antibodies, fueling segmental revenue growth.
Regionally, North America commands the largest industry share, leveraging advanced infrastructure and favorable regulations.
Meanwhile, Asia Pacific demonstrates the fastest CAGR due to supportive government initiatives and increasing outsourcing demand from domestic biopharma companies.
Biopharmaceutical Contract Manufacturing Market – Segmentation Analysis
To learn more about this report, Download Free Sample
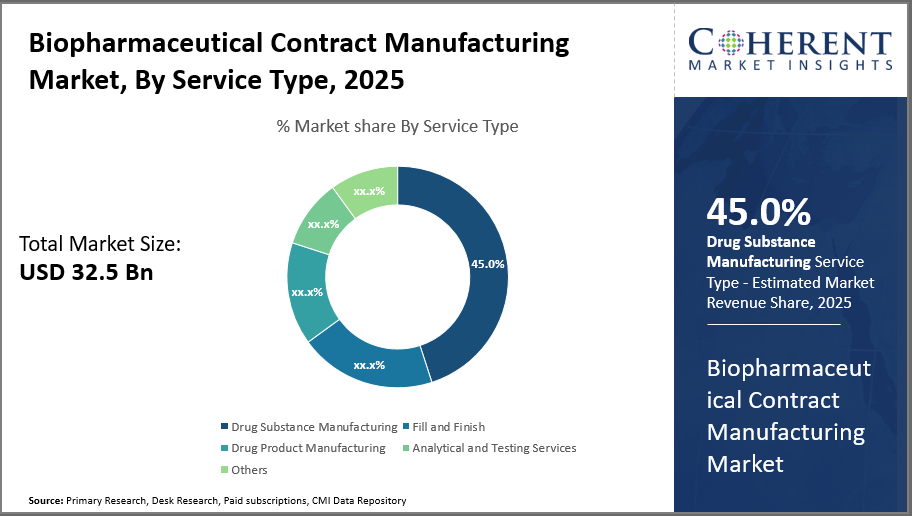
Biopharmaceutical Contract Manufacturing Market Insights, By Service Type
In terms of Service Type, drug substance manufacturing dominates the market share. Drug Substance Manufacturing leads due to its foundational role in biologics production, commanding nearly 45% of the service revenue pool. Growth here is largely driven by rising biologics pipelines and the need for advanced expression systems, including mammalian and microbial platforms, which support complex molecule synthesis. Further, the increasing need for precision therapeutics and sophisticated biologics, as well as the move away from conventional small molecule medications toward complicated big compounds that call for specialized production tools and knowledge, are driving this market.
Biopharmaceutical Contract Manufacturing Market Insights, By Therapeutic Area
In terms of Therapeutic Area, oncology dominating the market share. Oncology commands the largest portion due to the pipeline volume of monoclonal antibodies and cell therapies requiring specialized contract manufacturing services, accounting for nearly 38% of therapeutic segment revenue. The segment’s growth is supported by rising cancer incidence and innovation in immuno-oncology, which demand complex ADCs and CAR-T therapies. Biopharmaceutical companies are contracting with specialized manufacturers to produce complex treatments, particularly those for oncology, in order to comply with strict quality standards and regulatory requirements.
Biopharmaceutical Contract Manufacturing Market Insights, By Technology
In terms of Technology, mammalian cell culture dominates the market share. Mammalian Cell Culture represents approximately 52% share owing to its necessity in producing complex biologics like monoclonal antibodies and fusion proteins. This segment benefits from considerable investments in single-use bioreactors and continuous bioprocessing techniques that improve scalability and reduce contamination risks. The ability of mammalian systems to carry out intricate post-translational alterations is what makes them desirable for the safety and effectiveness of biologics, including recombinant proteins, monoclonal antibodies, and sophisticated therapies.
Biopharmaceutical Contract Manufacturing Market Trends
Recent years have seen critical emerging trends shaping the biopharmaceutical contract manufacturing market. The shift from traditional fermentation to advanced mammalian cell culture techniques underpins enhanced production yields and product complexity, as observed in 2024 capacity expansions by multiple CMOs. The increasing adoption of continuous bioprocessing has demonstrated improved efficiency, with a major U.S.-based CMO reporting a 15% reduction in production cycle times in 2025.
Furthermore, sustainability initiatives, such as minimizing single-use plastic waste in manufacturing operations, are gaining traction aligning with corporate responsibility goals. Another significant market shift is the rising prominence of emerging regions like Asia Pacific, where government incentives and lower operational costs attract market players, accelerating regional market share growth.
Biopharmaceutical Contract Manufacturing Market Insights, By Geography
To learn more about this report, Download Free Sample
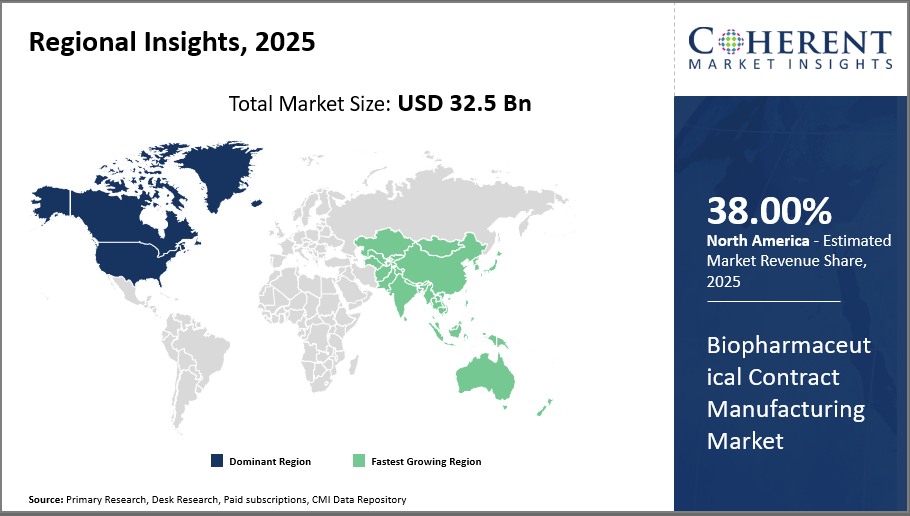
North America Biopharmaceutical Contract Manufacturing Market Analysis and Trends
Extensive biopharma infrastructure and regulatory harmonization in North America, which accounts for around 38% of the worldwide industry share, highlight the region's supremacy in the biopharmaceutical contract manufacturing market. Stable market expansion and a competitive advantage are guaranteed by the existence of important manufacturing centers in the United States as well as the adoption of cutting-edge technologies.
Asia Pacific Biopharmaceutical Contract Manufacturing Market Analysis and Trends
The Asia Pacific region is growing at the quickest rate, with a compound annual growth rate (CAGR) of over 12%. This growth is being driven by government incentives, the expansion of local biopharmaceutical businesses that outsource manufacturing, and increased investment in manufacturing capacities in China, India, and South Korea. Market participants' local strategic alliances and operations expansions contribute to this region's growth.
Biopharmaceutical Contract Manufacturing Market Outlook for Key Countries
United States Biopharmaceutical Contract Manufacturing Market Analysis and Trends
The U.S. market is crucial because of its strong biopharmaceutical R&D expenditures and concentration of top CMOs with expertise in mammalian cell culture and cutting-edge fill-finish services. Leading businesses that make large investments in capacity expansions and digital integration, such Catalent Inc. and Thermo Fisher Scientific, greatly boost the country's market revenue. Strict FDA rules maintain strict safety and quality requirements, which pushes contract manufacturers to constantly innovate. The United States is now a global leader in manufacturing thanks to this twin emphasis on compliance and technological adoption.
China Biopharmaceutical Contract Manufacturing Market Analysis and Trends
China’s biopharmaceutical contract manufacturing market has surged in recent years fueled by government policies encouraging biotechnology innovation and self-reliance. Large-scale capacity additions by domestic CMOs alongside increased adoption of single-use technologies typify this market. Chinese CMOs have attracted partnerships with multinational pharmaceutical companies seeking cost advantages and production scale, propelling the country’s market share in Asia Pacific. Furthermore, the rapid uptake of biosimilars is catalyzing business growth and infrastructure investments.
Analyst Opinion
The surge in biologics production capacity is a critical supply-side driver shaping the biopharmaceutical contract manufacturing market size. Recent data from 2024 indicates that capacity utilization rates of leading biologics CMOs have increased by approximately 15%, reflecting expansion investments that will secure supply continuity for upcoming biosimilar launches. For instance, a prominent contract manufacturer doubled its mammalian cell culture capacity in 2024 to meet growing demand.
Demand-side dynamics reveal significant growth in biosimilar imports, which accounted for nearly 22% of global biopharmaceutical imports in 2025. This influx is closely linked to cost advantages and shifting payer policies in developed regions, notably the United States and Europe, thereby enhancing market revenue opportunities for CMOs specializing in biosimilar production.
Micro-indicators such as oncology-focused contract manufacturing have gained momentum, constituting nearly 40% of biopharmaceutical manufacturing contracts in 2024. The expansion of immuno-oncology pipelines directly correlates with increased outsourcing activities, as evidenced by partnerships between leading pharmaceutical firms and specialized CMOs focusing on antibody-drug conjugates (ADCs).
Pricing dynamics continue to influence market growth strategies in the biopharmaceutical contract manufacturing space. A trend toward outcome-based pricing models is emerging, with some leading CMOs reporting up to 12% revenue growth through performance-linked contracts implemented in 2025. This shift aligns with pharmaceutical companies’ focus on cost containment and value-based healthcare delivery.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 32.5 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.3% | 2032 Value Projection: | USD 64.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Lonza Group Ltd., Samsung Biologics, Catalent Inc., Thermo Fisher Scientific, FUJIFILM Diosynth Biotechnologies, Wuxi Biologics, Boehringer Ingelheim, Patheon, AGC Biologics, Syngene International Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Biopharmaceutical Contract Manufacturing Market Growth factors
The biopharmaceutical contract manufacturing market revenue is strongly propelled by multiple key growth drivers. Primary among these is the robust increase in biopharmaceutical R&D pipelines, with more than 75% of novel biologics in development opting for outsourced manufacturing solutions to optimize capital expenditure. Advances in manufacturing technologies, including single-use bioreactors and continuous processing, further enable scalability and reduced contamination risks.
Additionally, stringent regulatory frameworks enacted in North America and Europe ensure high-quality standards that favor experienced CMOs, thereby amplifying market trust and growth. Market dynamics are also influenced by growing biosimilar acceptance, which fosters competitive pricing and expands market revenue, particularly in highly regulated economies.
Biopharmaceutical Contract Manufacturing Market Development
In May 2025, Lonza, a contract development and manufacturing organisation (CDMO), launched its new Design2Optimize platform, which will improve small molecule API manufacturing and process development. Optimized design of experiments (DoE), a statistical method for process and reaction condition optimization, is the foundation of the platform. The Design2Optimize platform is a proprietary model-based method that directs experimental setup according to ideal circumstances, with the aim of greatly speeding up the development timeline of small molecule APIs.
In May 2025, PCI Pharma Services, a worldwide contract development and manufacturing organization (CDMO) that specializes in cutting-edge biopharma treatments, has successfully acquired Ajinomoto Althea, a sterile fill-finish CDMO established in the US and a division of Ajinomoto, in Japan. The acquisition serves as the foundation for PCI's multi-year investment strategy, which includes sites in both Europe and the United States.
In July 2024, Sanofi acquired Vicebio, a biopharmaceutical company that is creating medicines to prevent deadly respiratory viral infections. As per the agreement, Vicebio stockholders will receive up to $1.6 billion, subject to customary circumstances. This amount includes a $1.15 billion upfront payment and $450 million in reimbursements for development and regulatory milestones.
Key Players
Lonza Group Ltd.
Samsung Biologics
Catalent Inc.
Thermo Fisher Scientific
FUJIFILM Diosynth Biotechnologies
Wuxi Biologics
Boehringer Ingelheim
Patheon
AGC Biologics
Syngene International Ltd.
Competitive strategies in recent years reflect aggressive capacity expansion and strategic acquisitions. For example, Samsung Biologics expanded its capacity by an additional 300,000 liters in 2024, bolstering its market share in mammalian cell culture. Similarly, Lonza’s acquisition of a precision manufacturing company in 2025 enabled the firm to integrate cutting-edge single-use technologies, enhancing both operational agility and contract win rates.
These market companies increasingly focus on end-to-end integrated manufacturing services to differentiate themselves from peers, leveraging digitalization and automation to improve turnaround times and reduce costs, thereby solidifying their competitive positioning.
Biopharmaceutical Contract Manufacturing Market Future Outlook
The market for biopharmaceutical contract manufacturing is expected to develop significantly due to the rising need for biologics, biosimilar, and sophisticated treatments such gene therapies, vaccines, and monoclonal antibodies. In order to take advantage of sophisticated manufacturing technologies, save operating costs, and concentrate on their core capabilities in research and development, pharmaceutical companies are increasingly outsourcing production to specialized contract manufacturing organizations (CMOs). In the production of biopharmaceuticals, innovations including automation, artificial intelligence, single-use systems, and continuous processing are improving quality, scalability, and production efficiency.
Furthermore, product development and commercialization timelines are being accelerated by strategic alliances and technology transfers between biopharma companies and CMOs. With the help of developing healthcare infrastructure and kind regulatory frameworks, geographic expansion in emerging countries is creating new prospects for contract manufacturing services globally. Outsourcing is becoming more and more popular as the industry adjusts to the complexity of cell and gene therapies, filling in infrastructural gaps and overcoming regulatory obstacles. All things considered, outsourcing trends, technical advancements, and growing access to innovative treatments worldwide will influence biopharmaceutical contract manufacturing in the future, making CMOs essential participants in the biopharma value chain.
Historical Development
For instance, in January 2020, iBioPharma Inc., a biotechnology company entered into collaboration with EdgePoint AI, a division of Mateon Therapeutics, to use EdgePoint’s proprietary artificial intelligence (AI)/blockchain-driven vision system for pharmaceutical manufacturing which was known as TrustPoint Fabric. It provided the highest level of compliance to the pharmaceutical industry’s standards for data integrity and enhanced automation capabilities which were expected to lower operating costs while improving quality for clients of iBio’s biologics contract development and manufacturing services.
In October 2019, Mesoblast, an Australian-based regenerative medicine company, and Lonza entered into an agreement for commercial manufacturing of Mesoblast’s allogeneic (off-the-shelf) cell therapy product candidate, remestemcel-L. The agreement enabled Lonza to expand its Singapore cGMP facilities and also anticipated the introduction of new technologies and process improvements which were expected to result in a significant increase in its manufacturing yields and efficiencies.
Sources
Primary Research interviews:
Quality Assurance & Regulatory Affairs Managers
Manufacturing Plant Managers
Databases:
SpringerLink
ScienceDirect
Wiley Online Library
Magazines:
BioPharm International
Pharma Manufacturing
European Pharmaceutical Review
Journals:
Bioprocess and Biosystems Engineering
Biotechnology Progress
Nature Biotechnology
Newspapers:
Financial Times (Pharma & Biotech coverage)
The Economic Times (Healthcare/Pharma)
Business Standard (Healthcare/Pharma)
Associations:
American Association of Pharmaceutical Scientists (AAPS)
European Federation of Pharmaceutical Industries and Associations (EFPIA)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients