Global biochips market is estimated to be valued at USD 13.34 Bn in 2025 and is expected to reach USD 37.73 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 16.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Increasing application of biochips in the field of proteomics, genomics and drug discovery can drive the market growth. Extensive R&D activities in the field of healthcare and growing demand for point-of-care testing and diagnostics can also boost demand for biochips during the forecast period. Advancements in microarray and microfluidic technologies can drive the global biochips market growth. Increasing government investments and funding for development of personalized medicine can drive the biochips market growth.
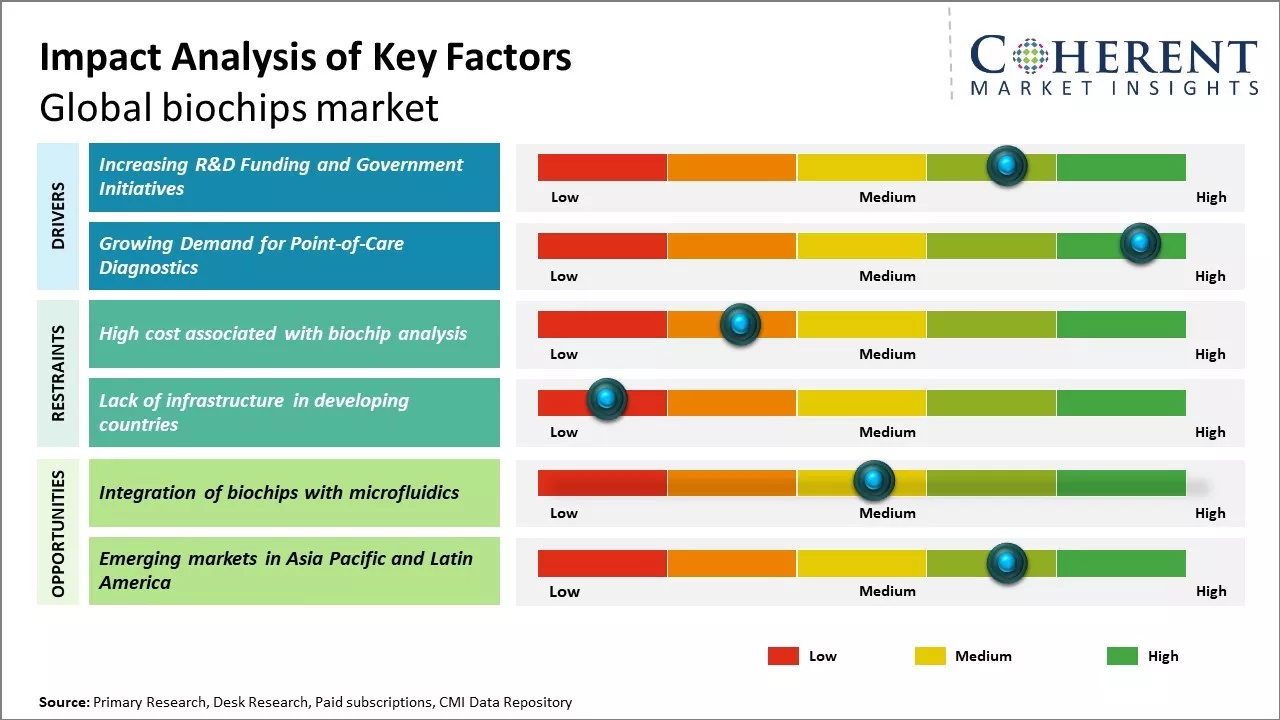
Increasing R&D Funding and Government Initiatives
Global biochips market growth is driven by increasing funding for life science research and development across both public and private sectors. Governments around the world recognize the profound potential of biochip technology to revolutionize healthcare diagnosis and treatment, unlock new opportunities in personalized medicine, and drive national innovation and economic growth. Thus, there has been increase in funding for research into novel biochip applications from both federal grants and public-private partnerships. Major economies like the U.S., China, Japan, Germany and India have all outlined ambitious strategic plans designating bioengineering and bio microsystems as national priorities. Alongside increased R&D funding, governments are incentivizing commercialization through tax holidays, reimbursement programs, and seed grants for startups. Rising international collaborations can also pool resources and accelerate the pace of development.
Get actionable strategies to beat competition: Download Free Sample
Growing Demand for Point-of-Care Diagnostics
Rising global need for more accessible, affordable and rapid point-of-care testing can drive the market growth. Traditionally, diagnostic testing has been centralized in large clinical laboratories requiring samples to be shipped, sometimes over large distances, for analysis. This centralized model comes with disadvantages like delays in receiving results, high costs of infrastructure, and inability to conduct real-time or continuous monitoring applications. However, miniaturized biochip technologies are now making "mini clinical laboratories" a reality through portable and easy-to-use diagnostic devices. This decentralized, point-of-care approach provide benefits to healthcare providers and patients. It allows for timely clinical decisions, enrollment in early treatment programs, and management of chronic conditions outside of hospital settings. Such on-site, real-time diagnostic capabilities are becoming increasingly important as populations age and face a growing chronic disease burden. There has been huge demand for convenient, low-cost biochip-based tests for applications such as infectious disease screening, cancer screening, non-communicable disease monitoring and personal genome analysis. This rising requirement for point-of-care testing worldwide can offer opportunities for companies involved in developing biochip-powered diagnostic solutions.
Key Takeaways from Analyst
Global biochips market growth is driven by rising demand for personalized medicine and point-of-care diagnosis. Advancements in microfluidics and microarray technologies can enable development of novel biochips with high sensitivity and multiplex detection capabilities. This can address various applications across healthcare, food and environment testing. North America currently dominates the biochips market, owing to presence of major players and focus on R&D activities for disease diagnostics. However, Asia Pacific is likely to emerge as fastest growing region due to increasing healthcare expenditures and focus on precision medicine in countries like China and India.
Miniaturization of detection system and incorporation of multiple biochemical assays in a single chip offer immense opportunities, however, high development costs can pose challenges for startups in this space. Lack of standardization and compatibility issues between different platforms also slows down commercial adoption of novel biochips. Furthermore, inefficient distribution networks in developing nations can hamper market growth. Strategic collaborations between players and regulatory bodies will be crucial to address these restraints. Strong government support for development of indigenous technologies will also boost emerging markets. Biochips market holds significant promise for innovations targeting point-of-care applications.
Market Challenges: High cost associated with biochip analysis
The high cost associated with biochip analysis can hamper the global biochips market growth. Biochip analysis requires expensive laboratory equipment, reagents and specialized research personnel, which makes the overall process quite costly. For example, initial set up and installation of a basic biochip analyzer can cost over US$ 100,000 and each experimental run on such equipment can be expensive. This heavy capital investment and operating expenses associated with biochip analysis makes it unaffordable for many small laboratories and research institutes especially in developing regions of the world. The high costs impose significant financial barriers and hurdles for widespread commercialization and clinical adoption of biochip technologies. Many diagnostic and genomic applications that could benefit from the high throughput analysis enabled by biochips are not viable due to the high per sample testing costs. Private healthcare providers are reluctant to invest in expensive biochip equipment as the return on investment may take significant time period. Patients also hesitate to opt for advanced but costly biochip diagnostics tests when equivalent options are available at lower prices through other conventional methods. Several startups and small biotech companies working on biochip-based solutions find it challenging to achieve economies of scale due to the high setup and operational expenses of initial prototyping.
Market Opportunities: Integration of biochips with microfluidics
The integration of biochips with microfluidics has the potential to significantly advance the global biochips market. Biochips combined with microfluidic technologies allow for complex biochemical reactions and analyses to be carried out in extremely small fluid volumes. This enables high-throughput molecular assays and analyses to be performed rapidly at the point-of-care. By miniaturizing full diagnostic labs onto a single chip sized device, microfluidic-biochip integration can make precision medicine more accessible and affordable worldwide. These lab-on-chip technologies could enable portable, easy-to-use, rapid and economic testing and screening for conditions like infectious diseases and chronic illnesses. Current research and development in this area aims to develop personalized disease screening, monitoring and drug response testing using saliva or blood samples of just microliters in volume. For instance, ongoing projects by NIH in 2021 are developing microfluidic-biochip devices capable of simultaneously detecting over fifty biomarkers from a single finger-prick of blood to comprehensively screen for heart disease and cancer risk. Such minimally invasive multi-analyte testing would prove transformative for healthcare especially in low-resource settings. It could facilitate widespread population-level screening campaigns and enable early detection when treatment options are most effective.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
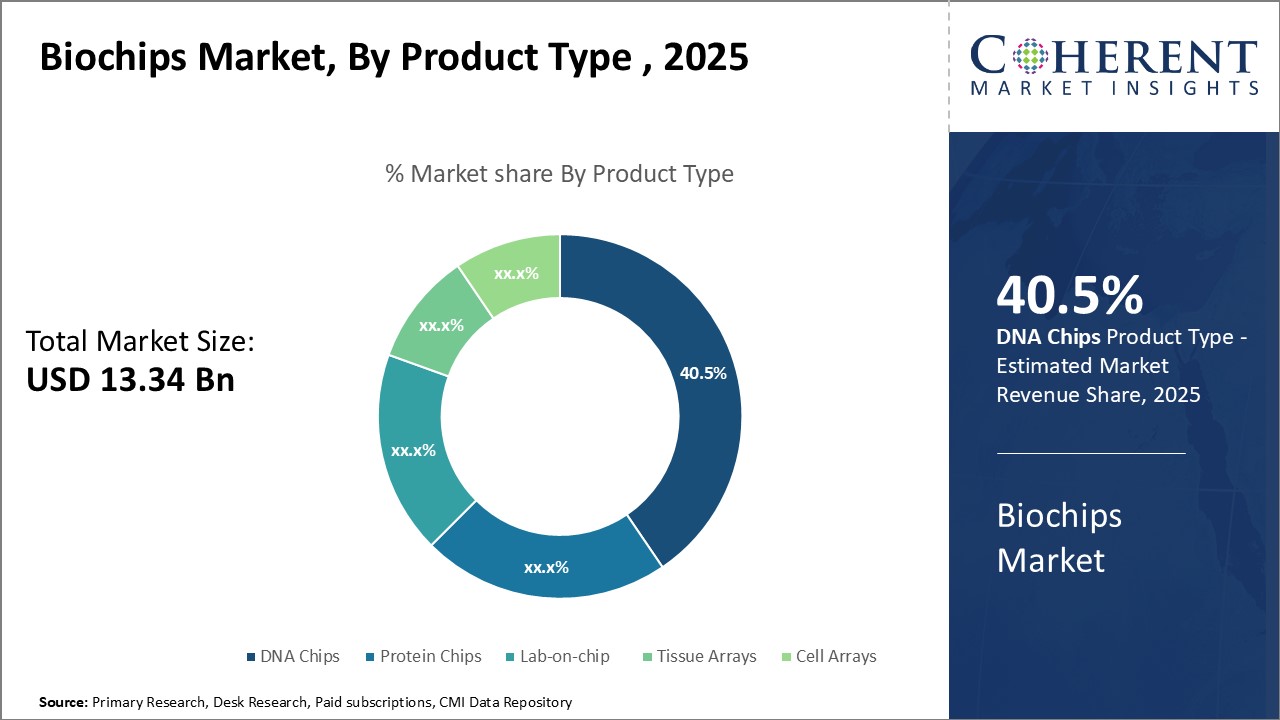
By Product Type- DNA Chips dominates due to wide range of applications
By product type, DNA chips segment is estimated to contribute the highest market share of 40.5% in 2025. DNA chips, also known as DNA microarrays, are a high-throughput screening technology that allows researchers to analyze a large number of target DNA sequences in parallel. DNA chips hold small DNA fragments called probes that complementarily bind to the target DNA or RNA sequences of interest. Using fluorescent tagging methods, researchers can analyze gene expression patterns or detect genetic mutations in thousands of samples simultaneously. The widespread use of DNA chips across genomics, genetic screening, and DNA/RNA analysis drives the segment growth in the global biochips market. DNA chips have revolutionized genome-wide association studies and enabled the discovery of genetic links to various diseases. Their ability to analyze hundreds or thousands of genes at once has accelerated genetic research and clinical diagnostics. DNA chips are extensively used in cancer research for tumor profiling, personalized therapy, and biomarker discovery. Their high sensitivity, precision, and throughput boosts their demand from biopharma companies and research organizations. The continuous technological advancements have further expanded applications of DNA chips. Developments like custom DNA microarrays have empowered researchers to design chips specific to their experiments. New DNA chips with higher probe densities and customized content allows for more in-depth investigations. Growing clinical requirements, such as non-invasive prenatal testing and pharmacogenomics, also boosts the need for innovative DNA chips.
By End User -Biotechnology and Pharmaceutical Companies dominates due to extensive R&D investments
By end user, biotechnology and pharmaceutical companies segment is estimated to contribute the highest market share of 35.12 % in 2025, primarily due to their massive investments in R&D. These organizations extensively utilize DNA chips, protein chips, and other biosensors for drug discovery, clinical trials, biomarker research, and toxicity screening. Biochips enable high-throughput screening of thousands of chemical compounds during lead identification stage of drug R&D. These facilitate analyzing interactions of drug candidates with targeted proteins, genes or cell receptors. This aids in accelerating early drug development with optimized candidates. In clinical research, biochips are widely used for pharmacogenomics to study individual variability in drug response and design more personalized therapies. Biopharma companies also invest heavily in developing customized biochips for proprietary use. For example, developing DNA or protein chips customized for biomarkers of certain disease areas like oncology. The generated results support clinical dossiers, companion diagnostic approvals and accelerate product commercialization. Biochips are gaining prominence in biomanufacturing for consistent quality control and testing during vaccine and biologics production.
Need a Different Region or Segment? Download Free Sample
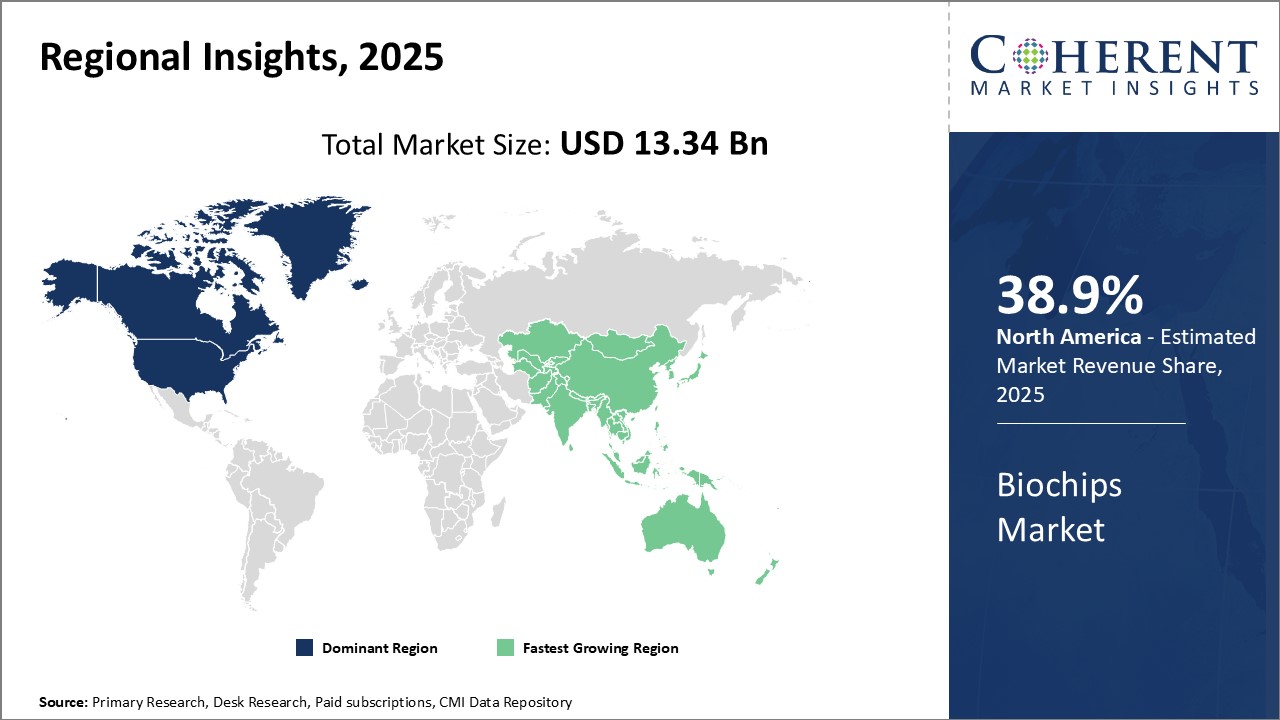
North America dominates the global biochips market with an estimated market share of 38.9% in 2025. The region is home to several large multinational biochip companies and startup biochip developers. It has a significant presence across the entire value chain from biochip manufacturing to testing and research. Many major technological innovations in biochip design and development originate from research institutions and universities located in the U.S. and Canada.
The region also has a highly supportive regulatory environment and strong protection for intellectual property rights, which encourages continued investment in biochip R&D. Countries like the U.S. provide competitive public funding for biochip projects. This attracts more private funding and partnerships between industry and academia. Thus, North America captures the largest share of biochip sales globally. It exports biochips to emerging markets and imports certain specialty biochips from other regions.
Asia Pacific is poised to be the fastest growing regional market for biochips. Rising healthcare expenditures and increasing focus on personalized medicine boosts biochip adoption. Countries like China, Japan, South Korea and India are rapidly building their domestic biochip production capabilities. For example, China's government supports its biochip manufacturers through targeted incentives and subsidies. This help reduce the region's reliance on biochip imports. Asia Pacific countries also provide attractive market opportunities due to their large and growing populations. Several multinational companies have established biochip manufacturing plants and R&D centers in Asia to cater to regional demand. Lower manufacturing costs compared to as North America and Europe allow Asia Pacific biochip makers to price their products competitively in the market. Continued expansion of the regional biohealthcare sector can drive the Asia Pacific biochips marketgrowth.
Biochips Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 13.34 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.0% | 2032 Value Projection: | USD 37.73 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
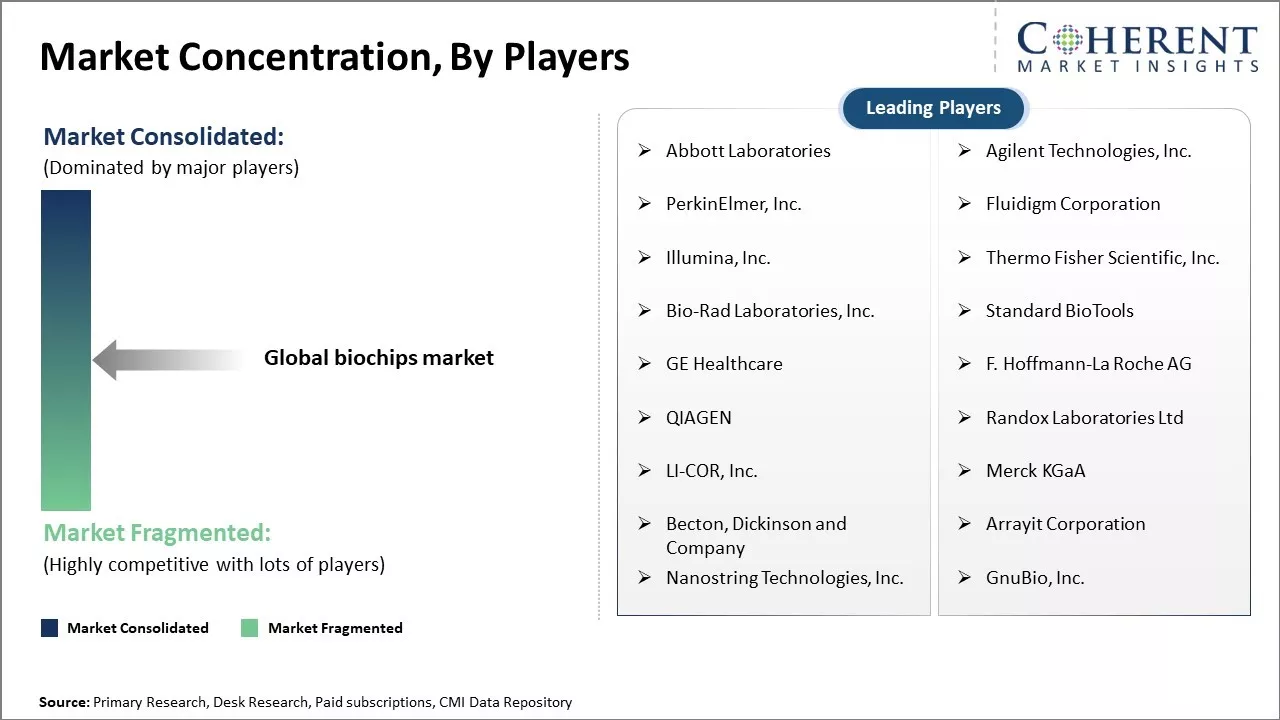
Abbott Laboratories, Agilent Technologies, Inc., PerkinElmer, Inc., Fluidigm Corporation, Illumina, Inc., Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Standard BioTools, GE Healthcare, F. Hoffmann-La Roche AG, QIAGEN, Randox Laboratories Ltd, LI-COR, Inc., Merck KGaA, Becton, Dickinson and Company, Arrayit Corporation, Nanostring Technologies, Inc., GnuBio, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global biochips market consists of microarrays or microchips that can detect and measure thousands of biological molecules or biological reactions simultaneously. This market involves the manufacture and sale of biochips/microarrays used for research in fields like genomics, proteomics, drug discovery, disease diagnostics, and other life science applications. The biochips analyze chemical reactions on a solid surface and help study biological systems in depth at the molecular level enabling advances in healthcare and biotechnology.
Product Type Insights (Revenue, USD Bn, 2020 - 2032)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients