Benign prostate hyperplasia (BPH) refers to the non-cancerous enlargement of prostate. The treatment option depends on various factors such as age of the patient, prostate gland size, and severity of the disease. According to an article published in Urologic Clinic of North America, 2016, BPH is a common problem that affects over 20% of men in the U.S., which is around 15 million men. The treatment option includes medication, surgery such as open prostatectomy, transurethral resection of the prostate (TURP), and minimally invasive therapies such as transurethral needle ablation (TUNA) and minimally invasive therapies such as laser therapy, radiofrequency ablation, implants and others.
Increasing demand for minimally invasive therapies is expected to boost growth of the benign prostatic hyperplasia surgical devices market
Minimally invasive surgeries have gained traction in the recent past, owing to various advantages such as significant reduction in postoperative pain, decreased recovery time, reduced hospital stays, lesser surgical scars, laparoscopic incisions being safer than open incisions, equivalent or better safety, and efficacy in abdominal and general surgeries as compared to open surgeries, and these surgeries may be less expensive than open surgeries. Moreover, these therapies offer one-time permanent solution rather than long-time dependent on medications. Currently, more than 60% and 20% of patients suffering from benign prostatic hyperplasia is on drugs and under observation. The minimally invasive therapies are focusing on these groups particularly and have also witnessed some success in attracting such customers.
For instance, implant, manufactured by NeoTract, Inc., (now acquired by Teleflex Incorporated), which entered U.S. market in 2013-14 saw exponential revenue growth in last 2 years, 2015 and 2016. In first two quarters of 2017 alone, the revenue generated crossed the revenue generated in last year (i.e. 2016) and is expected to perform similar in remaining two quarters of the year. By 2025, the revenue generated from implants may occupy nearly 40% share of the total benign prostatic hyperplasia surgical devices market, which is huge considering the recent introduction of products in market. There are other novel therapies, such as prostate artery embolization developed by Merit Medical Systems, Inc. and aquablation device developed by Procept BioRobotics, which demonstrated impressive therapeutic results in the clinical trials. These products with improved efficacy is expected to significantly enhance the adoption rate, in turn boosting the growth of the market.
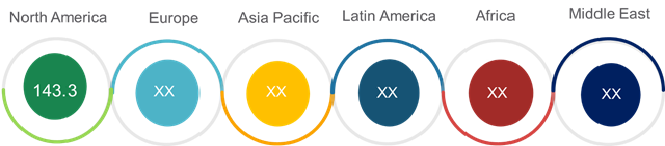
The global benign prostatic hyperplasia surgical devices market was valued at US$ 375.9 million in 2016 and is expected to witness a robust CAGR of 7.5% over the forecast period (2017 – 2025).
Figure 1. Global Benign Prostatic Hyperplasia Surgical Devices Market (US$ Mn), by Region, 2016
To learn more about this report, Download Free Sample
Increasing geriatric population is expected to drive the growth of benign prostatic hyperplasia surgical devices market
Benign prostatic hyperplasia is the most common prostate problem in men aged 50 years and above and the occurrence of the same tends to increase with age. According to the U.S. Census Bureau, 2015, among 7.3 billion people worldwide, an estimated 617.1 million people were aged 65 years and older and the number of geriatric population is expected to increase above 60% by year 2030 indicating the potential population base associated with lower urinary tract symptoms (LUTS) resulting due to benign prostatic hyperplasia. According to the Population Reference Bureau, numbers of American aged 65 years and above are expected to reach up to 98 million by 2060, more than double from 2014 and will constitute about one-fourth of the total population. This increasing aging population presents immense market opportunity for companies, specifically start-ups working on novel therapies such as ProVerum Limited, ProArc Medical and Zenflow, and Others.
Some major players operating in the benign prostatic hyperplasia surgical devices market are Olympus Corporation, Boston Scientific Corporation, Medifocus Inc., KARL STORZ SE & Co. KG, NxThera, Inc., ProArc Medical, Lisa Laser Products OHG, and Richard Wolf Gmbh.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients