Bacteriophage Therapy Market is estimated to be valued at USD 1,377.6 Mn in 2025 and is expected to reach USD 1,776.5 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 3.7% from 2025 to 2032.
Analysts’ Views on Global Bacteriophage Therapy Market:
Increasing investment funding by key market players is expected to drive market growth over the forecast period. For instance, in May 2021, Phico Therapeutics Ltd. (‘Phico’), a biotechnology company developing engineered phage technology, announced US$7.5 million (£7 million) in new investment by BGF, the U.K. based active growth capital investor. The funds were used to support the continued development of Phico’s SASPject technology platform and, in particular, the progression of its lead product, PT3.9, towards the clinic.
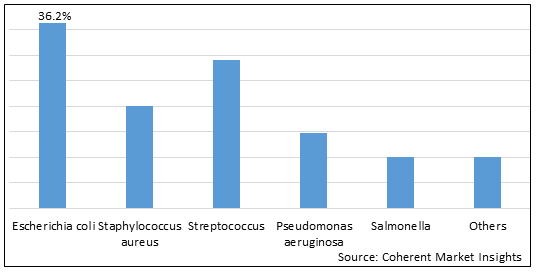
Figure 1. Global Bacteriophage Therapy Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Global Bacteriophage Therapy Market– Drivers
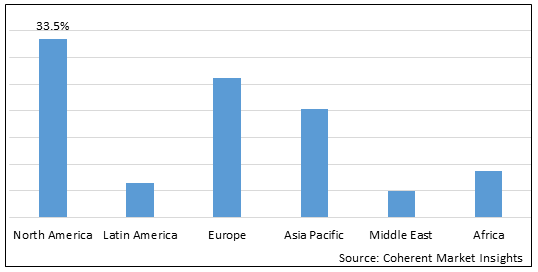
Figure 2. Global Bacteriophage Therapy Market Share(%), By Region, 2025
To learn more about this report, Download Free Sample
Global Bacteriophage Therapy Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global bacteriophage therapy market over the forecast period. North America is estimated to hold 33.5% of the market share in 2025. The global bacteriophage therapy market is expected to witness significant growth in the coming years, owing to increasing inorganic growth strategies such as investment funding by key market players to expand their product portfolio in the North America region. For instance, in October 2022, Adaptive Phage Therapeutics, Inc. (“APT) announced that the Defense Health Agency (DHA), a U.S. based joint, integrated combat support agency, had awarded an additional US$5 million to support the clinical development of APT’s adaptive bacteriophage (“phage”) therapy in the treatment of diabetic foot osteomyelitis (DFO). APT is evaluating the safety and efficacy of its precision phage-based therapy in the ongoing Phase 1/2 DANCE (DFO Adaptive Novel Care Evaluation) clinical trial.
Global Bacteriophage Therapy Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, are facing problems with the transportation of things from one place to another.
However, the COVID-19 pandemic had a positive impact on the global bacteriophage therapy market, owing to increased approval by regulatory authorities for the treatment of bacterial infections during COVID-19. For instance, in November 2020, Adaptive Phage Therapeutics (APT) announced the U.S. Food and Drug Administration (FDA) cleared the company’s Expanded Access for APT’s phage bank treatment for pneumonia or bacteremia/septicemia due to Acinetobacter baumannii, Pseudomonas aeruginosa or Staphylococcus aureus in COVID-19 patients. The FDA’s decision provides a new treatment option for critically ill COVID-19 patients with bacterial infections resistant to nearly all other available therapies. This COVID-19 patient population is rapidly increasing in line with the overall number of hospitalized COVID-19 patients.
Bacteriophage Therapy Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,377.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.7% | 2032 Value Projection: | USD 1,776.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Armata Pharmaceuticals, Inc., Eliava Biopreparations Ltd., Pherecydes Pharma, Intralytix, Inc., Phagelux, Inc., Nextbiotics, InnoPhage, Ltd, Locus Biosciences, Inc., TechnoPhage, Eligo Bioscience SA, Phagomed Biopharma GmbH., PhagePro, Inc., Adaptive Phage Therapeutics, Enbiotix, Inc., Intodeworld, Inc., BiomX Ltd., Phi Therapeutics, Fixed-phage Ltd., Micreos BV, ContraFect Corporation, OPTIPHARM Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Bacteriophage Therapy Market Segmentation:
The global bcteriophage therapy market report is segmented into targeted bacteria, disease indication, route of administration, distribution channel and region.
Among all the segments, the disease indication segment is expected to dominate the market over the forecast period, and this is attributed to increasing research and development activities by key market players to expand their product portfolio. For instance, in May 2022, Adaptive Phage Therapeutics, Inc. (“APT”) announced that the first patient has been dosed in its DFO Adaptive Novel Care Evaluation (DANCE) trial, a Phase 1/2 clinical study to evaluate the safety and efficacy of APT’s precision bacteriophage (“phage”) therapy in patients with Diabetic Foot Osteomyelitis (DFO).
Global Bacteriophage Therapy Market- Cross Sectional Analysis:
The increasing inorganic growth strategies, such as agreements by key market players, are expected to boost demand of the global bacteriophage therapy market in the North America region. For instance, in November 2021, Adaptive Phage Therapeutics, Inc. (APT) announced that it had entered into an agreement with the Antibacterial Resistance Leadership Group (ARLG), a group conducting clinical trials, to support a multi-center Phase 1b/2, randomized, double-blind, placebo-controlled trial assessing the safety and microbiological activity of a single dose of bacteriophage therapy in cystic fibrosis subjects colonized with Pseudomonas aeruginosa.
Global Bacteriophage Therapy Market: Key Developments
Global Bacteriophage Therapy Market: Key Trends
Global Bacteriophage Therapy Market: Restraint
Global Bacteriophage Therapy Market - Key Players
Major players operating in the global bacteriophage therapy market include Armata Pharmaceuticals, Inc., Eliava Biopreparations Ltd., Pherecydes Pharma, Intralytix, Inc., Phagelux, Inc., Nextbiotics, InnoPhage, Ltd, Locus Biosciences, Inc., TechnoPhage, Eligo Bioscience SA, Phagomed Biopharma GmbH., PhagePro, Inc., Adaptive Phage Therapeutics, Enbiotix, Inc., Intodeworld, Inc., BiomX Ltd., Phi Therapeutics, Fixed-phage Ltd., Micreos BV, ContraFect Corporation, OPTIPHARM Co., Ltd.
*Definition: Phage therapy (PT) is also called bacteriophage therapy. It uses viruses to treat bacterial infections. Bacterial viruses are called phages or bacteriophages. They only attack bacteria; phages are harmless to people, animals, and plants. Phage therapy mainly utilizes obligately lytic phages to kill their respective bacterial hosts while leaving human cells intact and reducing the broader impact on commensal bacteria that often results from antibiotic use.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients