Automated Endoscopy Reprocessor Market– Automatically Disinfecting Reprocessed Endoscopes
Automated Endoscopy Reprocessors (AERs) are extensively used in healthcare settings for reprocessing of endoscopes such as duodenoscopes and endoscope accessories, to disinfect between uses. AERs disinfect reusable endoscopes by exposing their interior channels and outside surfaces to chemical solutions.
Figure 1. Global Automated Endoscopy Reprocessor Market, By Product Type, 2017
To learn more about this report, Download Free Sample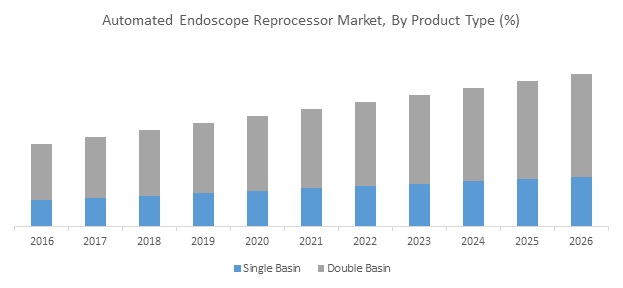
Source: Coherent Market Insights Analysis (2018)
Increasing number of gastrointestinal (GI) diseases demanding endoscopic procedures are expected to drive the automated endoscope reprocessor market growth. According to a National Institute of Health data 2014, around 20 million GI endoscopic procedures are performed annually in the U.S. Endoscopy professionals are at risk of chemicals, body fluids contamination of patients, muscle skeleton injuries, and exposure of radiation. According to International Journal of Surgery report published in 2014, the rate of contracting an infection, during a gastrointestinal endoscopic procedure is around one in 1.8 million operations. However, this infection rate may be underrated due to incomplete surveillance, asymptomatic infections, under-reporting, and infections with a long incubation period.
AERs replace few manual steps involved in endoscope reprocessing. AERs have basins to allow endoscopes to be submerged in high-level disinfection (HLD) solution. After HLD cycle, AERs automatically cleans the reprocessed endoscope with water to remove toxic HLD solution residues followed by forced air to dry the endoscope channels and prevent growth of waterborne pathogenic microorganisms during storage.
The global automated endoscopy reprocessor market size was valued at US$ 845.3 million in 2017 and is expected to witness a robust CAGR of 6.7% over the forecast period (2018–2026).
Figure 2. Global Automated Endoscopy Reprocessor Market Value (US$ Mn), by Region, 2017
To learn more about this report, Download Free Sample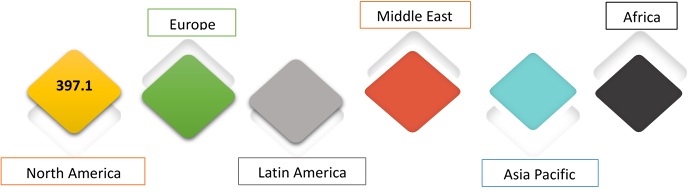
Source: Coherent Market Insights Analysis (2018)
Increasing adoption of endoscope reprocessors in healthcare settings to augment the market growth
Increasing adoption rate of endoscope reprocessors in hospitals for infection prevention and control is expected to fuel the global automated endoscopy reprocessor market revenue. AERs can enhance reliability and consistency of endoscope reprocessing by standardizing several important reprocessing steps, thereby reducing the possibility of human error. The use of AERs reduces exposure of personnel to harmful chemical germicides, thereby minimizing health problems attributed to reprocessing of endoscopes.
Moreover, rising government concerns pertaining to patient safety and increasing healthcare infrastructure are factors contributing to rise in global automated endoscopy reprocessors market size. For instance, in 2015, the Centers for Disease Control and Prevention (CDC) requested the Healthcare Infection Control Practices Advisory Committee (HICPAC) to issue guidelines for improvement of facility-level training to ensure competency for endoscope reprocessing devices.
Increasing focus on product innovation is expected to support growth of the market
Various changes required in AER and endoscope design will facilitate to further reduce the risk of transmission of infections related to endoscopy. The design of flexible GI endoscopes was traditionally focused on enhanced function and performance and not on ease of cleaning and HLD. In some endoscopes such as the duodenoscope, the complex design presents a particular challenge to cleaning and HLD. The FDA has asked AER manufacturers to validate reprocessing instructions, mainly for AERs that reprocess duodenoscopes.
Several product recalls from leading manufacturers requires product upgrade and manufacturing of innovative products. For instance, in 2016, the FDA signed off on Cantel's Medivators (Advantage Plus and DSD Edge) and Steris (System 1E Liquid Chemical Sterilant Processing System) automated endoscope reprocessors that are labeled for use with duodenoscopes. In the same year, the organization also signed off on a new version of a duodenoscope from Olympus.
In addition to this, in November, 2015, the FDA ordered Custom Ultrasonics to recall all of its AERs from health care facilities due to the firm’s violations of the FD&C Act, applicable regulations, and the Consent Decree. Continuous product recalls due to stringent government regulations is expected to adversely affect the market growth, however, the opportunity to manufacture advanced products in harmony with government norms can open opportunities.
Major players operating in the global automated endoscopy reprocessor market include Cantel Medical Corp., Johnson & Johnson, Laboratoires Anios, Olympus Corporation, Steelco SpA, Steris Plc., Getinge Group, Hoya Group, and Metall Zug AG.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients