Auto-injector is a medical device which is used for injecting oneself with a single, preloaded dose of a drug. It is consists of a spring-loaded syringe which gets activated when the device is pushed firmly against the body. Auto-injector devices are useful for the rapid administration of a particular drugs and antidotes. An auto-injector has a drug cartridge with an embedded needle for subcutaneous or intramuscular injection, which is usually painless. Then, drugs are slowly delivered by the auto-injector across a large area in the muscle, which increases the drug absorption and the drug effects. Epinephrine auto-injector is a type of auto-injector widely used for people at risk of anaphylaxis. They can also be used by those who have not been medically trained. Auto-injectors are often used in the military to protect personnel from chemical warfare agents.
The global auto-injectors market is estimated to be valued at US$ 4,497.7 million in 2022 and is expected to exhibit a CAGR of 18.0% during the forecast period (2022-2030).
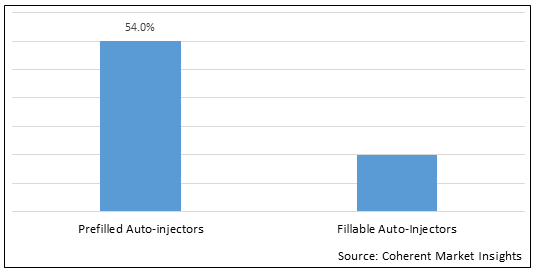
Figure 1. Global Auto-injectors Market Share (%), by Product Type, 2022
To learn more about this report, Download Free Sample
Global Auto-injectors Market - Driver
Increasing inorganic strategies such as agreement is expected to boost the growth of global auto-injectors market. For instance, in May 2022, Stevanato Group, a manufacturer and distributor of drug containment, announced that they had signed an agreement with Owen Mumford Ltd., a manufacturer of medical device, for its Aidaptus auto-injector. This agreement will bring value to customers, matching world-class device expertise with premium manufacturing capabilities. Aidaptus auto-injector is a two-step, single use auto-injector with a versatile design that accommodates both 1 mL and 2.25 mL prefilled glass syringes in the same base device.
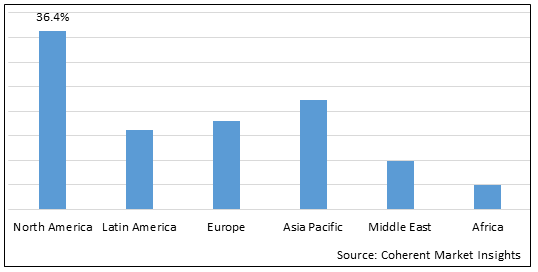
Figure 2. Global Auto-injectors Market Value (US$ Million), by Region, 2022
To learn more about this report, Download Free Sample
Global Auto-injectors Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global auto-injectors market due to the increasing development of vaccines and treatment against COVID-19. For instance, in May 2022, the European Medicine Agency (EMA) approved Vaxzevria in the European Union (EU) as a third dose booster in adults. Thus, the increasing approvals of vaccines will demand the injectable drug delivery devices like needles and syringes.
Auto-injectors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 4,497.7 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 18.0% | 2030 Value Projection: | US$ 16,953.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Becton, Dickinson and Company, Sanofi S.A., Takeda Pharmaceutical Company Limited, Xeris Biopharma Holdings Inc., BlackHagen Design, GSK plc., Genentech, Inc., AstraZeneca, Owen Mumford Ltd., Stevanato Group, Jabil Inc., Pfizer, Inc., Mylan N.V., Novartis AG, Bayer AG, Janssen Pharmaceuticals, Inc., Antares Pharma, Eli Lilly and Company, Amgen Inc., Rafa Laboratories Ltd., Halozyme, Inc., Biogen, and Teva Pharmaceutical Industries Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Auto-injectors Market: Key Developments
Adoption of inorganic growth strategies such as product launch is expected to drive the global auto-injectors market. For instance, in May 2022, Jabil Inc., a medical device company, announced that they had launched the Qfinity auto-injector. It is a self-administered, reusable, and modular solution for subcutaneous (SC) drug that is delivered at a lower cost.
Global Auto-injectors Market: Restraint
Increasing number of product recalls by regulatory authorities such as the U.S. Food and Drug Administration. For instance, in September 2021, a trulicity auto-injector, which is manufactured by Eli Lilly & Company, a pharmaceutical company, was recalled by the U.S. Food and Drug Administration due to labelling error. The auto-injector device was labeled as 0.75 mg/0.5 mL as it was actually contained 1.5 mg/0.5 mL of product. The recall affects 119,539 boxes of Trulicity 0.75 mg/0.5 mL single-dose pens, four pens per box (NDC 0002-1433-80), from lot D396436C.
Global Auto-injectors Market - Key Players
Major players operating in the global auto-injectors market include Becton, Dickinson and Company, Sanofi S.A., Takeda Pharmaceutical Company Limited, Xeris Biopharma Holdings Inc., BlackHagen Design, GSK plc., Genentech, Inc., AstraZeneca, Owen Mumford Ltd., Stevanato Group, Jabil Inc., Pfizer, Inc., Mylan N.V., Novartis AG, Bayer AG, Janssen Pharmaceuticals, Inc., Antares Pharma, Eli Lilly and Company, Amgen Inc., Rafa Laboratories Ltd., Halozyme, Inc., Biogen, and Teva Pharmaceutical Industries Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients