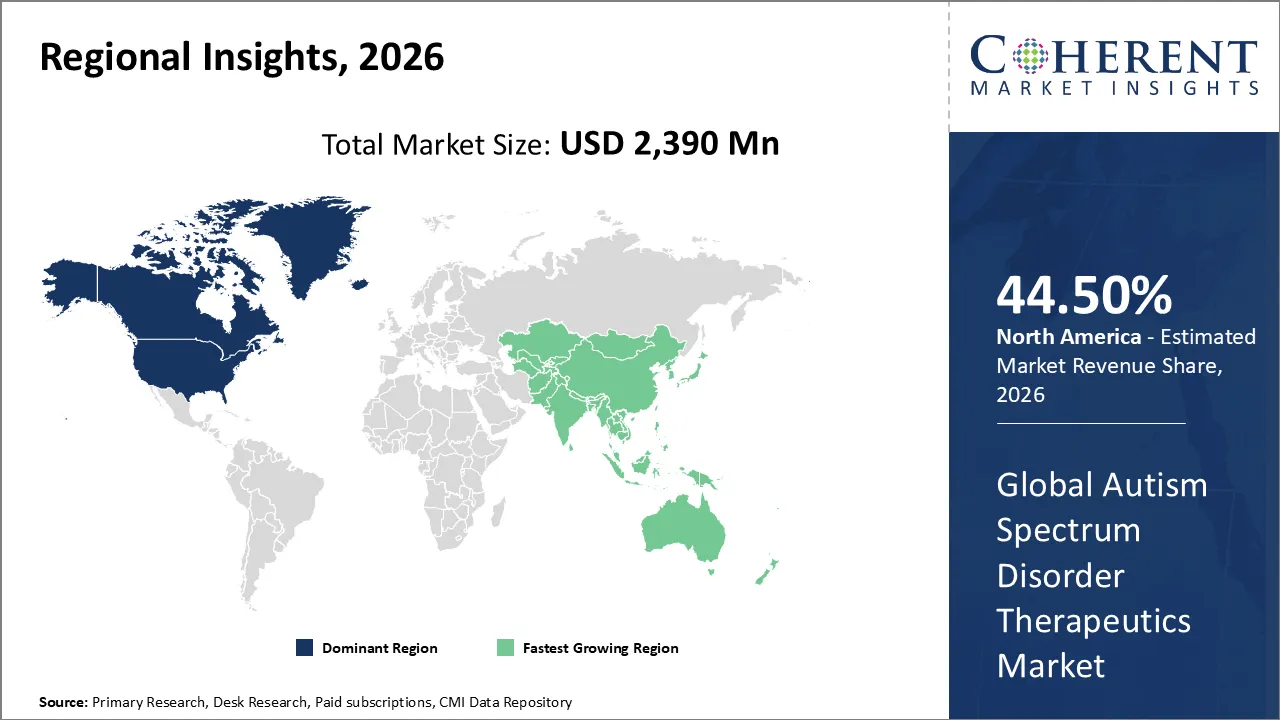
The Autism Spectrum Disorder Therapeutics Market is estimated to be valued at USD 2,390 Mn in 2026 and is expected to reach USD 3,761 Mn by 2033, growing at a compound annual growth The rate (CAGR) of 6.7% from 2026 to 2033.
The Autism Spectrum Disorder Therapeutics Market is advancing significantly by its expanding role in personalized medicine, digital healthcare integration, and the management of complex comorbidities. The increasing diagnostic accuracy and tightening clinical standards for evidence-based interventions are expected to drive the market growth over the forecast period.
ASD therapeutics are a multidisciplinary category of treatments and are mainly composed of pharmacological agents such as antipsychotics and stimulants and behavioral interventions formed from neurodevelopmental research. They are integral to the healthcare, education, and social service systems due to their impact on functional communication and behavioral outcomes.
The demand for specialized ASD care is increasing across pediatric hospitals, specialized clinics, home-based settings, and school-based programs. These developments are strengthening patient engagement, thus enabling data-driven monitoring, and improving the overall efficiency of long-term care models.
|
Current Event |
Description and the Impact |
|
Regulatory Advances and Policy Changes |
|
|
Technological Innovations in Therapeutics |
|
|
Regional Epidemiological and Awareness Trends
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of age group, the child segment contributes the highest share of 49.60% in the market in 2026. This leadership stems from the early intervention paradigm, as doctors emphasize that treatment yields better outcomes during the period of peak brain elasticity. The expansion of screening programs within schools and pediatric offices has resulted in a substantial increase in diagnoses among children aged 3 to 11. The government funded educational programs and insurance mandates prioritize services for minors, thus ensuring continuous access to care. As a result, there is an increasing demand for behavioral therapies and medication tailored to pediatric developmental needs. Parents and providers focus heavily on this age group to maximize long-term success.
For instance, in December 2024, the Israeli Medical Cannabis Agency renewed its authorization for SciSparc Ltd. to proceed with the SCI-210 clinical trial focused on children diagnosed with autism spectrum disorder (ASD). SCI-210 is a patented formulation that combines cannabidiol, or "CBD," with CannAmideTM.
In terms of drug type, the risperidone segment contributes the highest share of 41.40% in 2026 of the market. This is because the FDA specifically approves it to treat irritability and aggression in patients. Clinicians use it to manage severe behavioral disturbances such as tantrums and self-injurious behaviors, even though it does not address core social communication deficits. Its long-standing safety profile in pediatric populations drives continued segment growth. The manufacturers are coming up with multiple formulations, including oral solutions and orally disintegrating tablets, to accommodate patients with sensory sensitivities or swallowing difficulties. These options help families and healthcare providers maintain risperidone as a cornerstone of pharmacological intervention.
In terms of distribution channel, the hospital pharmacies segment contributes the highest share of 51.10% in 2026 of the market due to the complex nature of the initial diagnosis and treatment. In most cases, physicians commence pharmacotherapy in specialized psychiatric or pediatric departments in order to ensure stringent clinical monitoring and precise titration. These environments enable systematic surveillance for metabolic complications associated with the use of high-potency antipsychotic agents, including risperidone. In addition, multidisciplinary care teams within hospital settings streamline the transition from the initial diagnostic evaluation to the fulfillment of specialized prescriptions. This centralized approach allows for more effective management of complex patient needs than is typically possible in other settings.
To learn more about this report, Download Free Sample
North America has remained the dominant region with 44.50% in 2026 of the global Autism Spectrum Disorder Therapeutics Market over the past decade. The growth is owing to a well developed healthcare infrastructure and high diagnostic rates in the region. The robust government support via initiatives like the Autism Cares Act provides essential funding for research and specialized services. In addition, favorable reimbursement policies and the presence of major pharmaceutical players speed up the adoption of novel drug therapies. These factors combine to create a mature market environment that prioritizes early intervention and advanced clinical care for patients and families.
For instance, in November 2025, Liquid Biosciences and Ignite Biomedical, Inc. have announced the discovery of biomarkers that may assist in diagnosing and treating autism spectrum disorder (ASD). This achievement highlights their ongoing focus on developing diagnostic and treatment prediction tests in areas with significant unmet needs and also signals the start of Ignite's foray into drug development.
The Asia Pacific region stands out as the fastest-growing segment in the market. The rapidly improving healthcare infrastructure and rising public awareness are fueling this growth in nations like China, India, and Japan. The governments in the region are implementing standardized screening programs in schools with an aim to identify neurodevelopmental disorders at earlier stages. In addition, the experiencing of specialized pediatric clinics and the entry of global pharmaceutical firms in these emerging nations increase the variability of treatment options. A large population base and increasing healthcare expenditure ensure that this region will continue to gain significant global market share over the forecast period.
For instance, in August 2025, the angel stem cell treatment for autism spectrum disorder (ASD), developed by Biostar Stem Cell Technology Research Institute, has received approval from the Japanese Ministry of Health, Labor, and Welfare (MHLW).
The US dominates the ASD therapeutics market owing to the consistently high diagnosis rates. According to the estimates by the CDC nearly 1 in 36 children has been identified with ASD. This epidemiological scale creates sustained demand for advanced therapeutic solutions and multidisciplinary care models. In addition, a robust telehealth infrastructure, broad insurance reimbursement, and ongoing federal funding through the Autism Cares Act are continuing to speed up market growth. The country is an ideal launch market for new stimulants and antipsychotics, supported by efficient regulatory pathways and high levels of patient adoption. The rapid integration of digital health tools and telemedicine has substantially improved treatment accessibility for families throughout the region.
For instance, in January 2024, Jaguar Gene Therapy announced that its Investigational New Drug (IND) application for JAG201 has been approved by the U.S. Food and Drug Administration (FDA). JAG201 is a gene therapy aimed at treating a hereditary variant of autism spectrum disorder (ASD) and Phelan-McDermid syndrome (PMS).
China is the principal contributor to growth in the Asia Pacific, driven by sustained government prioritization of mental health system modernization. The Chinese market is experiencing consistent expansion fueled by increasing public awareness and the development of specialized pediatric departments in urban centers. Authorities are currently implementing standardized screening programs in schools to improve early identification rates for neurodevelopmental disorders. Despite the dominance of antipsychotic medication, stimulants are emerging as a key growth area for treating co-occurring hyperactivity and inattention. Additionally. The establishment of a national pilot program for special education signifies a major shift toward long-term support frameworks.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 2,390 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 6.7% | 2033 Value Projection: | USD 3,761 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
F. Hoffmann-La Roche AG, Neurim Pharmaceuticals, Inc., Yamo Pharmaceuticals LLC, Servier Laboratories Ltd., Otsuka Holdings Co. Ltd., Curemark LLC, Oryzon Genomics S.A., GW Pharmaceuticals Plc., Teva Pharmaceutical Industries Ltd., Zynerba Pharmaceuticals Inc., Stalicla SA., Novartis AG, Pfizer, Inc., and Bristol Myers Squibb. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The global surge in the prevalence of autism spectrum disorder is the primary catalyst for the rapid expansion of the specialized therapeutics market. This increase is largely driven by refined diagnostic criteria and a significant rise in public awareness, which have collectively identified a much larger patient population than in previous decades. Earlier diagnosis rates are propelling substantial and growing demand for interventions that improve long-term functional outcomes.
The pharmaceutical companies of biotechnology firms are shifting their focus from generic symptom management to the development of targeted therapies that address the core neurological pathways of the disorder. The integration of digital therapeutics and personalized medicine is enhancing the market by providing highly individualized care solutions. A growing patient base coupled with a robust pipeline of innovative treatments is driving continual growth in the autism therapeutics sector. These factors are fueling sustained investment and development worldwide.
The Autism Spectrum Disorder (ASD) therapeutics market is demonstrating sustained expansion, supported by rising diagnostic penetration, increasing prevalence across pediatric and adolescent populations, and continued investment in neurodevelopmental research. Epidemiological data from public health agencies indicate a steady increase in diagnosed ASD cases, which is directly translating into higher demand for both pharmacological and adjunctive therapeutic interventions.
Pharmacological treatment remains primarily focused on managing associated symptoms such as irritability, hyperactivity, anxiety, and sleep disturbances. Antipsychotics and stimulants continue to be widely prescribed, supported by clinical evidence showing statistically significant improvements in behavioral scores and functional outcomes in controlled studies. At the same time, the clinical pipeline is expanding, with multiple late-stage and mid-stage assets targeting core and associated ASD symptoms through differentiated mechanisms of action.
Regionally, mature healthcare systems continue to lead in treatment adoption due to established diagnostic frameworks, specialist availability, and reimbursement structures. Emerging markets are gradually contributing to incremental growth as awareness initiatives, early screening programs, and access to pediatric neurological care improve.
From a strategic perspective, the market is transitioning from a limited, symptom-management-centric landscape toward a more diversified therapeutic ecosystem. Continued clinical trial activity, regulatory engagement, and real-world evidence generation are expected to shape treatment standards, positioning the ASD therapeutics market as an area of long-term clinical and commercial significance.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients