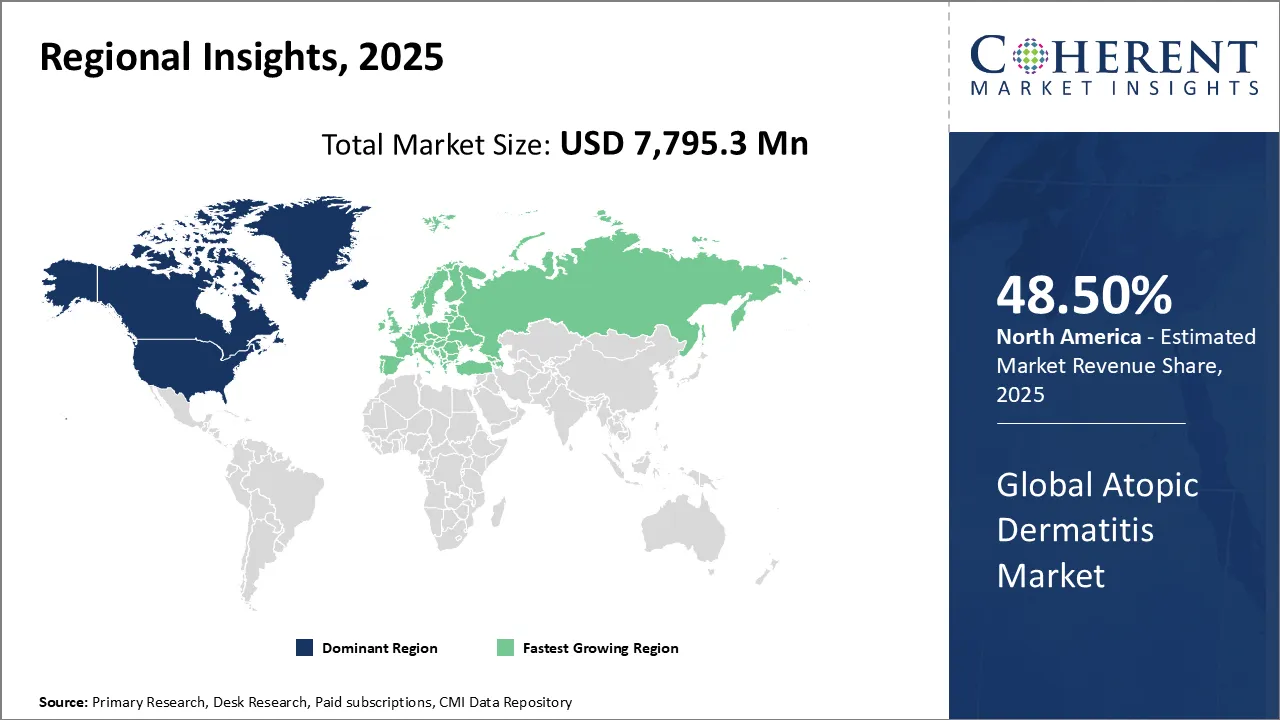
The Global Atopic Dermatitis Market was valued at USD 7,795.3 Mn in 2025 and is forecast to reach a value of USD 14,941.2 Mn by 2032 at a CAGR of 9.7% between 2025 and 2032.
The global atopic dermatitis market is experiencing strong growth due to the increasing prevalence of atopic dermatitis and increasing awareness among people. Moreover, increase in focus on the development of novel therapeutics is also offering lucrative opportunities. However, factors such as adverse effects of certain therapeutic drugs and treatment cost variations limit patient access are expected to hamper the market growth.
|
Event |
Description and Impact |
|
Advanced Biologic Therapies Pipeline Development |
|
|
US-China Trade Relations and Pharmaceutical Supply Chain Tensions |
|
|
European Regulatory Framework Evolution |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Atopic dermatitis (AD) poses a major healthcare burden. Biologics drive 60% of costs but treat only 15–20% of patients. Reimbursement varies widely: CMS (US) covers biologics under Medicare/Medicaid with cost-sharing; EMA and national HTA bodies (EU) assess cost-effectiveness; Asia-Pacific markets like Japan and Australia offer 70–90% coverage. Insurance impact is significant—US HDHPs, PPOs, and HMOs differ in copays, deductibles, and prior authorizations. Access is shaped by coding frameworks (ICD-10, CPT, HCPCS), with biologics requiring strict authorization and high out-of-pocket costs despite broad regulatory support.
AD affects 7–10% of adults and up to 25% of children. Prescriber surveys (2024) show first-line reliance on topical corticosteroids (TCS)—hydrocortisone for sensitive areas, triamcinolone for body lesions, clobetasol/betamethasone for severe flares. Topical calcineurin inhibitors (TCIs), including tacrolimus and pimecrolimus, are favored as steroid-sparing options, especially for facial and pediatric cases. Barrier repair is integral: ceramide-based moisturizers (CeraVe, Cetaphil) are preferred, with prescription creams (EpiCeram, MimyX) and urea-based products used for enhanced repair. Prescribers cite efficacy, safety, and patient suitability as drivers of therapeutic choice.
By drug class, corticosteroids account for the largest share of the atopic dermatitis market in 2025. Their widespread use is attributed to their effectiveness in reducing inflammation and managing flare-ups, making them the mainstay of treatment for atopic dermatitis. Topical corticosteroids remain the first-line therapy prescribed by dermatologists worldwide.
In December 2024, Galderma announced that the U.S. Food and Drug Administration (FDA) has approved Nemluvio® (nemolizumab) for use in patients aged 12 and older who have moderate-to-severe atopic dermatitis. This treatment is to be used alongside topical corticosteroids (TCS) and/or calcineurin inhibitors (TCI) when the condition is not sufficiently managed with prescription topical therapies alone.
In terms of administration, the topical segment is expected to dominate the market over the forecast period, supported by its role as the primary method of managing atopic dermatitis. The ease of application, direct delivery to affected skin, and wide availability of topical formulations reinforce its leadership.
The oral segment, however, is anticipated to register notable growth in the near future, driven by rising cases of moderate to severe atopic dermatitis. For such cases, oral corticosteroids like prednisone are frequently prescribed, supporting expansion of this segment.
By prescription type, the over-the-counter (OTC) segment is projected to hold the largest market share during the forecast period. The dominance of OTC products stems from their accessibility, affordability, and growing use for preventing flares and managing mild symptoms of atopic dermatitis. Widely available across global retail channels, OTC drugs such as moisturizers, antihistamines, and hydrocortisone creams are increasingly being adopted by patients.
The prescription segment continues to play a crucial role in the management of moderate to severe cases, but the ease of access and preventive role of OTC medications underpin their leading position in the overall market.
To learn more about this report, Download Free Sample
North America is projected to hold the largest share of 48.50% in the global atopic dermatitis market in 2025, supported by rising incidence of the disease, increasing public awareness, and government-led campaigns to improve skin health education. The U.S. remains the key driver in the region, where atopic dermatitis poses not only physical challenges but also significant emotional and quality-of-life burdens.
Europe is also anticipated to witness strong growth in the atopic dermatitis market, underpinned by the high prevalence of the disease and proactive efforts from both government bodies and industry players. Increasing healthcare initiatives and availability of advanced therapies have made the region a key growth hub.
According to the European Medicines Agency (EMA), up to 7% of adults in Europe suffer from atopic dermatitis, with around 30% of cases classified as moderate to severe. Notably, one in four adults report onset of the disease during adulthood, highlighting its broad and lasting impact across age groups. These factors, combined with supportive healthcare infrastructure and rising treatment adoption, are expected to drive robust market expansion across Europe.
The U.S. dominates the North American market due to its high patient population, advanced healthcare infrastructure, and rising adoption of both prescription and OTC treatments. Increasing awareness campaigns and support from organizations such as the National Eczema Association are contributing to greater treatment compliance and early diagnosis.
For example, recent public health initiatives emphasize reducing the stigma associated with visible skin conditions and improving patient access to dermatology specialists. This is expected to enhance adoption of both traditional corticosteroid therapies and newer biologic treatments.
Germany represents a significant share of the European market, supported by strong healthcare infrastructure and high per-capita healthcare spending. The country has seen a rise in cases of moderate to severe atopic dermatitis, which is driving demand for advanced therapies including biologics and targeted immunomodulators.
Government reimbursement policies and proactive clinical research further strengthen the German market outlook. Ongoing collaborations between pharmaceutical companies and academic research centers are expected to accelerate the availability of innovative treatment options.
The U.K. market is supported by rising awareness campaigns, particularly among pediatric populations where the prevalence of atopic dermatitis is high. National Health Service (NHS) initiatives and patient advocacy programs play a key role in promoting early treatment and reducing disease burden.
Additionally, the U.K.’s participation in clinical trials for novel biologics and small-molecule inhibitors positions the country as a hub for innovation in atopic dermatitis care. Increasing investment in dermatology research and digital health solutions for chronic skin conditions is expected to further support market growth.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7,795.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.7% | 2032 Value Projection: | USD 14,941.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Novartis AG, Nestle, LEO Pharma AS, Encore Dermatology Inc., GlaxoSmithKline PLC, Allergan PLC, AbbVie Inc., Regeneron Pharmaceuticals Inc., and Bausch Health Companies Inc., among others. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
One of the key factors expected to augment the growth of the global atopic dermatitis market over the forecast period is the rise in prevalence of atopic dermatitis across the globe. Atopic dermatitis, also known as eczema, is a chronic disease that causes inflammation, redness, and irritation of the skin. It is a common condition that usually begins in childhood; however, people of all ages live with a condition. According to the World Health Organization (WHO) Global Burden of Diseases initiative, more than 230 million people across the world have atopic eczema, and this number is expected to increase during the forecast period.
Another factor which is driving the growth of the global atopic dermatitis market is the increasing awareness among people about atopic dermatitis. Atopic eczema is one of the most prevalent skin diseases in the world. Thus, governments worldwide and players in the market are launching novel initiative/campaign to raise atopic dermatitis awareness. For instance, Atopic Eczema Community comes together on September 14th (every year) to raise awareness for the disease, speak up about the burden it has on patients and caregivers, and to recognize the need for care and treatment that is reflective of the multi-dimensional nature of the disease.
Increase in focus on the development of novel therapeutics is expected to offer significant growth opportunities for players in the global atopic dermatitis market. For instance, players in the market are focusing on launching novel therapeutics owing to rise in burden of atopic dermatitis. On the World Atopic Dermatitis Day, Sanofi and Regeneron announced the launch of new, global grants initiative seeking proposals for grassroot solutions to help solve some of the greatest challenges impacting people living with atopic dermatitis (AD).
With the rise in prevalence of atopic dermatitis, the demand for safe and effective AD treatment. As a result, players in the market are focusing on developing and launching novel therapeutics in the market. Left untreated, eczema can lead to severe symptoms and increased risk of developing secondary infections. In severe long-term cases, untreated childhood eczema may interfere with growth and development.
Topical corticosteroids have been the mainstay of treatment for atopic dermatitis over the years. Corticosteroids are used as pain relief medicines for inflammation in the body. Corticosteroids are known to reduce itching, redness, swelling, and allergic reactions. Clinical trials have also shown that topical corticosteroids are safe and effective to treat atopic dermatitis flare-ups.
In July 2025, LEO Pharma received FDA approval for ANZUPGO® (delgocitinib) cream (20 mg/g) in the U.S. for moderate-to-severe chronic hand eczema in adults for whom topical corticosteroids are not advisable or have been inadequate.
The global atopic dermatitis market is highly competitive. This is attributed to the rise in burden or prevalence of atopic dermatitis across the globe, as a result, players in the market are focusing on launching novel products in the market.
Some of the key players in the global atopic dermatitis market are
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients