Newborn screening has evolved from a simple urine or blood screening test to a more comprehensive and complex screening system capable of detecting over 50 different conditions such as Critical Congenital Heart Defect (CCHD), Hearing Screening, Sickle Cell Disease, and Maple Syrup Urine Disease (MSUD). Newborn screening tests measure a number of markers in infant’s blood that can be either decreased or increased if an infant has certain diseases. Currently, conditions such as cystic fibrosis (CF), congenital hypothyroidism, phenylketonuria (PKU), and around 22 other metabolic conditions that affect protein or fat metabolism can be diagnosed through newborn screening tests.
The Asia pacific newborn screening market is estimated to be valued at US$ 308.9 million in 2022 and is expected to exhibit a CAGR of 12.9% during the forecast period (2022-2030).
Figure 1. Asia Pacific Newborn Screening Market Share (%), by End User, 2022
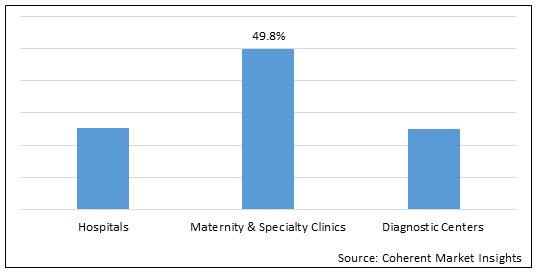
To learn more about this report, Download Free Sample
Increasing government initiatives is expected to drive growth of the Asia Pacific newborn screening market
Increasing engagement programs by various government healthcare regulatory bodies for newborn screening and its deployment for wider population base are expected to drive growth of the market during the forecast period. For instance, in January 2022, Trivitron, medical devices company, informs that implementation of newborn screening at the national level will help to identify the infants who need urgent medical care at a much larger scale. Newborn screening is a program that facilitates the early detection of several genetic, endocrine, and metabolic disorders.
Furthermore, in March 2018, Under National Rural Health Mission, significant progress had been made in reducing mortality in children. Whereas, there is an advance in reducing child mortality there is a dire need to improving survival outcome. This would be reached by early detection and management of conditions that were not addressed comprehensively in the past. Indian Government started the RASHTRIYA BAL SWASTHYA KARYAKRAM (RBSK) in 2019 for the early diagnosis of genetic, endocrine and metabolic disorders. Rashtriya Bal Swasthya Karyakram (RBSK) is an important initiative aiming at early identification and early intervention for children from birth to 18 years to cover 4 ‘D’s namely defects at birth, deficiencies, diseases, development delays including disability.
Asia Pacific Newborn Screening Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 308.9 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 12.9% | 2030 Value Projection: | US$ 815.2Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic plc, Agilent Technologies, Waters Technologies Corporation, AB SCIEX, Bio-Rad Laboratories, Covidien PLC, GE Life Sciences, Masimo Corporation, Natus Medical Inc., PerkinElmer Inc., Trivitron Healthcare Pvt. Ltd., and ZenTech S.A. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Asia Pacific Newborn Screening Market Share (%), by Countries, 2022
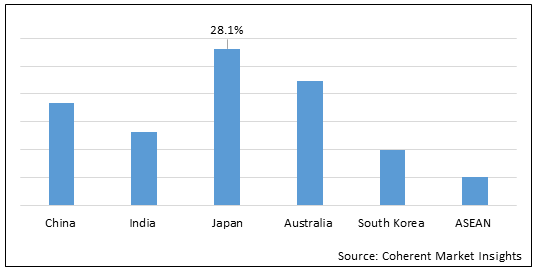
To learn more about this report, Download Free Sample
Increasing use of advanced technology for newborn screening is expected to drive the market over the forecast period.
Technological advancements in tandem mass spectrometry, colorimetric analysis test, fluorescence analysis test, enzyme or liquid chromatography, and various immunological assays and other analysis and high adoption rate of newborn screening with new technology are factors that are expected to drive growth of the market. In October 2018, LifeCell International, India’s leading stem cell bank and mother & baby diagnostics company, launched RightStart, the world’s first integrated DNA testing for newborn screening to detect over 50 medical conditions.
Asia Pacific Newborn Screening Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, medical screening, nutritional supplements, etc. by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the Asia pacific newborn screening market, owing to the disruption in healthcare services. For instance, in March 2022, according to data published by the National Center for Biotechnology Information, global COVID-19 pandemic has presented extraordinary disruption to healthcare services and exposed them to numerous challenges. Newborn screening (NBS) programmes were also affected; however, scarce data exist on the impact of COVID-19 on NBS. COVID-19 impacted the NBS programs at least partially in most of the responding countries. Majority of the screening centers experienced a broad spectrum of difficulties and were affected to a greater extent in the second wave of the pandemic. Delays and unreliability in the postal service as well as flight cancellations caused delays in sample transport to screening centers. The provision of laboratory equipment and reagent supplies was also affected. The number of staff available to perform screening was reduced due to infection, quarantine or reassignment to COVID-19 wards, sometimes resulting in complete closure of screening laboratories and the need to send the samples to other centers for analysis.
Asia Pacific Newborn Screening Market: Key Developments
In June 2022, PerkinElmer Inc., global corporation focused in the business areas of diagnostics, life science research, food, environmental and industrial testing will host a three-day scientific event exploring the latest advances in newborn screening programs and related technologies. Organized around the theme of ‘Learn, Share and Develop,’ PerkinElmer’s Newborn Screening World View summit will feature more than 20 presentations from participants across 11 countries. Each day, presentations and Q&A sessions scheduled will focus on a different topic, which will include: Newborn screening around the world – discussions around new and expanding programs worldwide, and novel ways to overcome common challenges. Leading the newborn screening conversation – expert advice on optimizing workflow efficiencies, throughput and accuracy, as well as opportunities to expand newborn screening panels. The future of newborn screening – conversations around next generation sequencing, molecular screening and expanding screening techniques and ideas.
Asia Pacific Newborn Screening Market: Restraint
The major factors that hinder growth of the Asia pacific newborn screening market include lack of improvement in healthcare infrastructure and trained professionals. Newborn screening is still not widely adopted in Asia Pacific region and requires more development and awareness. Countries in Asia Pacific region have high population and less number of trained professionals. High population with low and middle-income level and poor quality health services are hindering growth of the market.
Key Players
Major players operating in the Asia Pacific newborn screening market include Medtronic plc, Agilent Technologies, Waters Technologies Corporation, AB SCIEX, Bio-Rad Laboratories, Covidien PLC, GE Life Sciences, Masimo Corporation, Natus Medical Inc., PerkinElmer Inc., Trivitron Healthcare Pvt. Ltd., and ZenTech S.A.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients