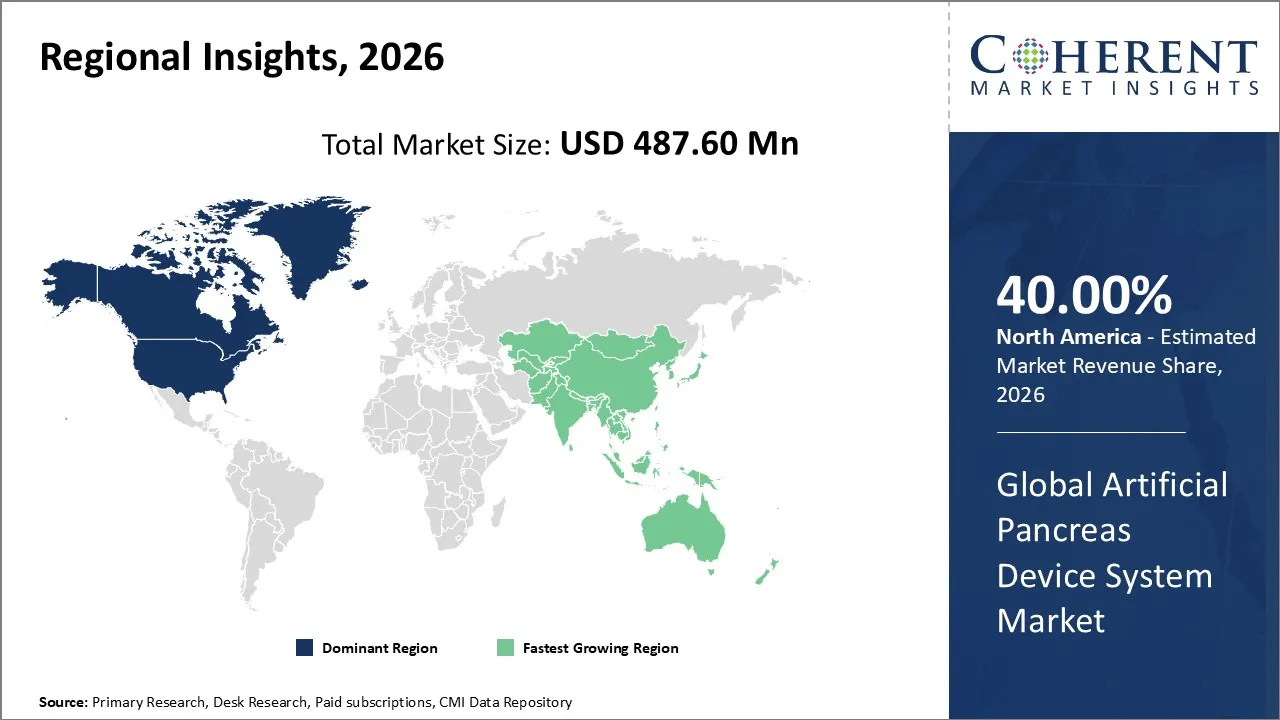
Artificial pancreas device system market is estimated to be valued at USD 487.60 Mn in 2026 and is expected to reach USD 1,634.2 Mn in 2033, exhibiting a compound annual growth rate (CAGR) of 18.9% from 2026 to 2033.
Administering insulin on a daily basis can be cumbersome for type 1 diabetes (T1D) patients. Devices such as insulin pumps, pens, and jet injectors have made managing diabetes less stressful by replacing painful insulin delivery devices such as needles and syringes. Moreover, organizations such as the Juvenile Diabetes Research Foundation (JDRF) have collaborated with industry players such as Medtronic, Inc., Johnson & Johnson, and Tandem Diabetes Care, Inc. to develop innovative insulin and glucose monitoring systems/devices.
The artificial pancreas device system (APDS) includes an insulin pump, sensor, transmitter, and a receiver. MiniMed 530G by Medtronic, Inc., was the first artificial pancreas device system (APDS) approved by the U.S. FDA. This threshold suspend device system enables temporary suspension of insulin delivery when the glucose level is lower than the threshold level.
|
Current Event |
Description and its Impact |
|
FDA Regulatory Evolution and Global Harmonization |
|
|
Rising Global Diabetes Prevalence and Healthcare Costs |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Partnership |
Devices/Systems |
Impact |
|
Abbott + Beta Bionics |
Dual glucose-ketone sensor + iLet Bionic Pancreas |
First fully automated insulin dosing system |
|
Abbott + Medtronic |
FreeStyle Libre CGM + MiniMed 780G pump + InPen |
Expanded patient options with sensor + pump integration |
|
Abbott + Tandem Diabetes Care |
Dual sensor + Mobi insulin pump |
Enhanced safety with ketone detection |
|
Abbott + Sequel Med Tech |
Dual sensor + twiist AID system |
Expanded ecosystem beyond major players |
|
MicroTech Medical (China) |
PanCares closed-loop APDS |
Supported by NMPA innovation pathway |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of technology, the treat-to-range/control-to-range segment is expected to hold 47.8% share of the market in 2026. These devices balance automation and safety, adjusting insulin to maintain glucose within a safe range. Their proven clinical effectiveness, regulatory acceptance, and patient trust make them the preferred choice over threshold suspend and treat-to-target systems.
For instance, in June 2025, A recent study on Tandem’s t:slim X2 insulin pump with Control-IQ technology shows improved glucose management in pregnant women with type 1 diabetes. The system, part of Treat-to-Range/Control-to-Range technology, increased time-in-range and reduced highs, reinforcing its dominance in artificial pancreas device systems as a safe, effective solution.
In terms of distribution channel, the hospitals & clinics segment is expected to lead the market with 62% share in 2026. Patients rely on professional guidance, training, and monitoring when adopting advanced pancreas systems. Strong reimbursement policies, physician recommendations, and integration into established healthcare infrastructure ensure hospitals remain the primary channel for widespread adoption.
For instance, in September 2025, The NHS has rolled out a new generation artificial pancreas for pregnant women with type 1 diabetes, delivered through hospitals and clinics. This initiative enhances glucose control, ensuring safer pregnancies. Professional monitoring, physician guidance, and reimbursement support reinforce hospitals and clinics.
To learn more about this report, Download Free Sample
North America is expected to lead the artificial pancreas device system market with 40% share in 2026, due to rising type 1 diabetes prevalence, advanced healthcare infrastructure, strong reimbursement policies, and rapid FDA approvals.
For instance, in January 2025, Beta Bionics raised $204 million to launch its IPO, funding development of the iLet Bionic Pancreas. This closed-loop system integrates continuous glucose monitoring with automated insulin delivery, reducing patient burden and improving outcomes.
Asia Pacific is anticipated to be the fastest growing region, due to rising diabetes prevalence, growing awareness of advanced therapies, supportive healthcare policies, and rapid adoption of digital health technologies. Expanding infrastructure and affordability drive growth in the region.
For instance, in November 2025, Medtrum launched TouchCare, the world’s smallest closed-loop automated insulin delivery system, featuring the first 300-unit tubeless insulin pump. Integrating continuous glucose monitoring with smart algorithms, it automatically adjusts insulin to maintain safe glucose levels.
The U.S. Artificial Pancreas Device System market is highly demanding in 2026 due to rising type 1 diabetes prevalence, advanced healthcare infrastructure, strong reimbursement policies, and rapid FDA approvals. Continuous innovation, patient preference for closed-loop automation, and investment in medtech drive growth, positioning the U.S. as the global leader.
For instance, in June 2025, Analysts predict Beta Bionics plans to launch its patch pump in 2027, expanding its iLet Bionic Pancreas portfolio. The tubeless design integrates with continuous glucose monitoring and algorithms to automate insulin delivery, enhancing convenience and outcomes.
China’s Artificial Pancreas Device System market is highly demanding in 2026 due to rising diabetes prevalence, government focus on chronic disease management, and expanding healthcare expenditure. Regulatory support from NMPA, rapid adoption of CGM and patch pumps, and local innovation like MicroTech’s PanCares system drive strong growth and patient demand.
For instance, in March 2025, MicroTech Medical’s Hong Kong Exchange filing highlights progress in diabetes care. Its PanCares closed-loop artificial pancreas system passed NMPA’s innovation review, with registration applications underway in China and Europe. Complementary products include AiDEX CGM and Equil Patch Insulin Pump.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 487.60 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 18.9% | 2033 Value Projection: | USD 1,634.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic Plc., Medtrum Technologies Inc., Tandem Diabetes Care, Inc., Johnson & Johnson, Beta Bionics, Inc., Bigfoot Biomedical, Insulet Corporation, Pancreum, Inc., TypeZero Technologies, Inc., DreaMed Diabetes Ltd, and Inreda Diabetic BV |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
With over 500 million people worldwide living with diabetes, the burden continues to rise, particularly for type 1 diabetes patients who require lifelong insulin therapy. This growing population drives the artificial pancreas device system market demand, as patients increasingly seek automated solutions to reduce the daily burden of glucose monitoring and insulin dosing. Closed-loop systems offer improved quality of life, better glycemic control, and reduced complications, making automation a critical response to the escalating diabetes prevalence globally.
Advances in closed-loop systems integrating continuous glucose monitors (CGMs), insulin pumps, and sophisticated algorithms are transforming diabetes care. Hybrid and bi-hormonal designs enhance accuracy and safety, while AI-powered devices from innovators like MicroTech and Beta Bionics predict insulin needs more effectively. These breakthroughs significantly increase the artificial pancreas device system market share, as patients and healthcare providers adopt cutting-edge technologies. Innovation not only improves outcomes but also accelerates adoption, positioning APDS as a cornerstone of modern diabetes management worldwide.
Regulatory approvals play a pivotal role in accelerating commercialization of advanced diabetes technologies. The FDA’s streamlined review process in the U.S. and NMPA’s innovation pathways in China enable faster market entry for closed-loop systems. These frameworks reduce barriers for companies like Beta Bionics and MicroTech Medical, ensuring patients gain quicker access to life-changing devices. This regulatory momentum strengthens confidence in the artificial pancreas device system market forecast, projecting robust growth across developed and emerging regions.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients