Antithrombin Market Size and Forecast – 2025 – 2032
The Global Antithrombin Market size is estimated to be valued at USD 1.65 billion in 2025 and is expected to reach USD 2.78 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032.
Global Antithrombin Market Overview
The antithrombin market involves therapeutic and diagnostic products derived from plasma or recombinant sources used to regulate blood coagulation. Antithrombin concentrates are administered to patients with congenital or acquired deficiencies, preventing conditions such as deep vein thrombosis, pulmonary embolism, and disseminated intravascular coagulation. Products include lyophilized powders and injectable formulations, available in both human plasma-derived and recombinant variants.
Recombinant antithrombin offers advantages in purity, viral safety, and consistent potency, making it preferred in clinical settings. Beyond therapeutic applications, antithrombin is also used in research and diagnostic assays for coagulation monitoring. Manufacturers are focusing on enhanced stabilization techniques, longer-acting formulations, and expanded indications for cardiovascular and surgical patients, driving product diversification in the global anticoagulant therapy landscape.
Key Takeaways
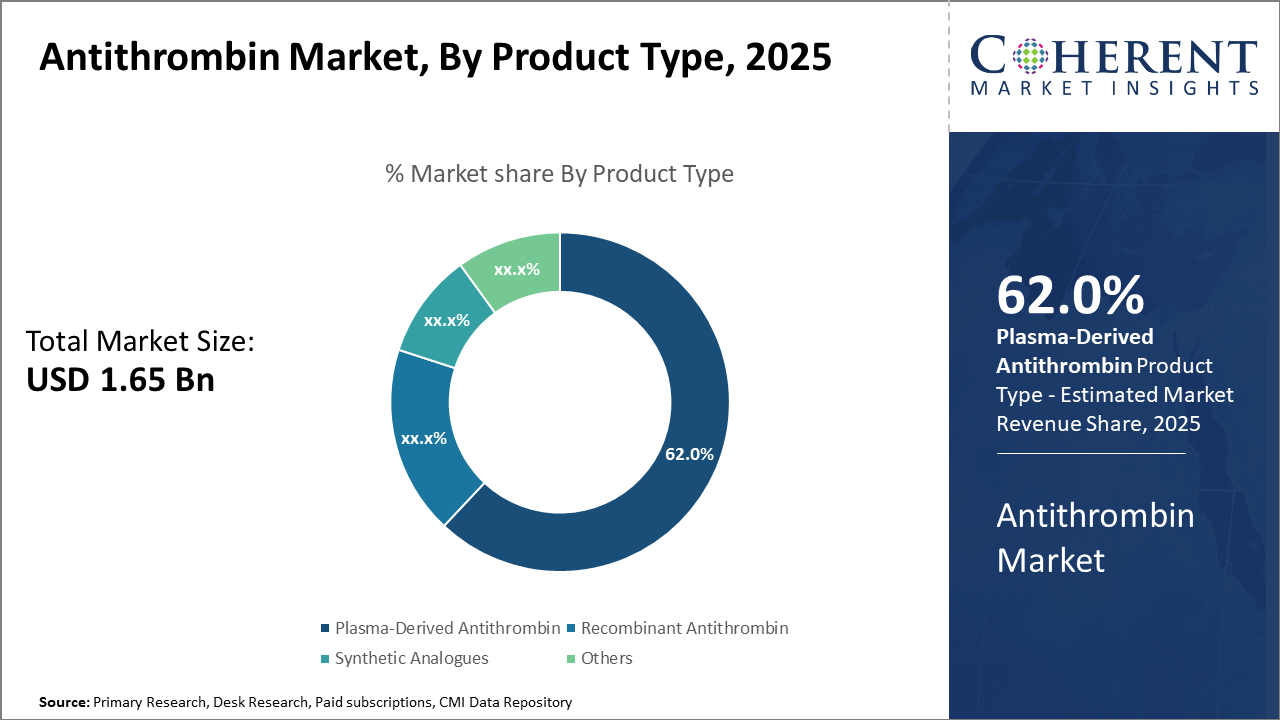
In the product type segment, plasma-derived antithrombin dominates the market share with 62%, attributed to its wide clinical acceptance and established efficacy profile, while recombinant antithrombin demonstrates the fastest growth due to innovation-driven adoption.
Within applications, cardiovascular surgery remains the largest segment, supported by increasing surgical cases and clinical preference, whereas emerging application areas like hemodialysis are rapidly expanding.
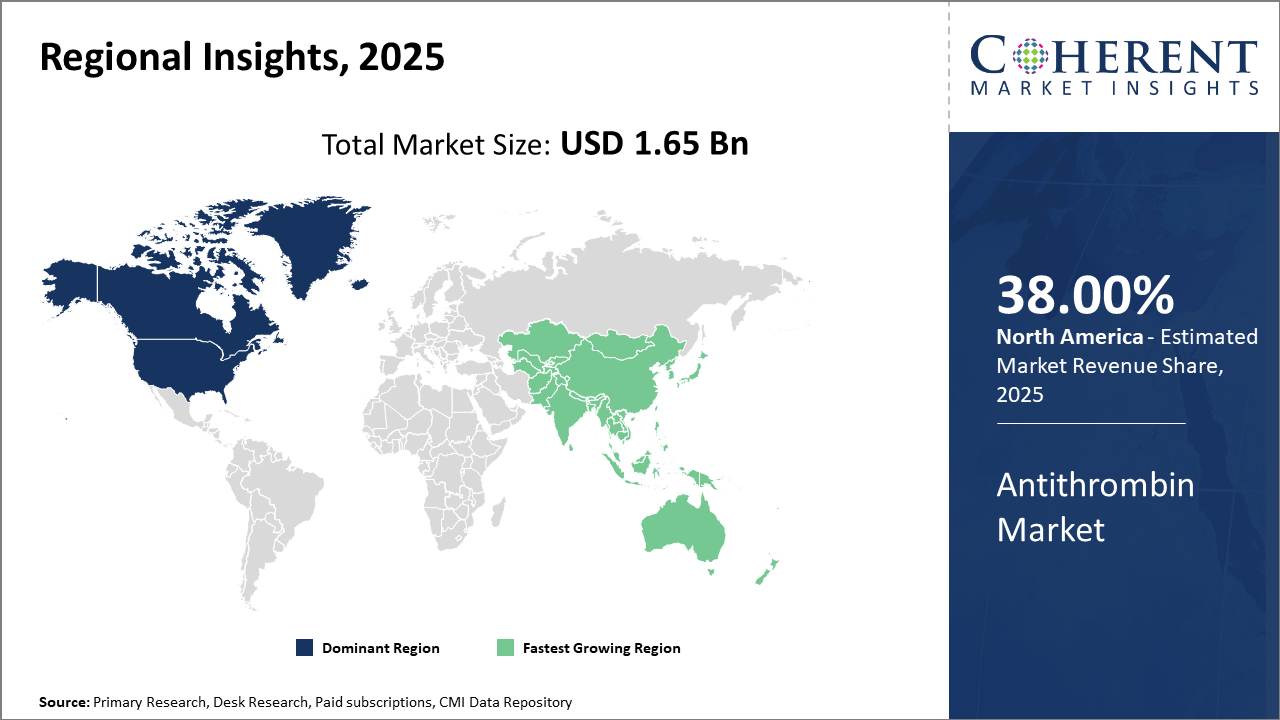
Regional analysis highlights North America’s dominance with approximately 38% market share, driven by robust healthcare infrastructure and comprehensive regulatory support. Asia Pacific registers the highest CAGR, fueled by rising healthcare expenditure, growing patient pool, and expanding plasma collection networks.
Antithrombin Market Segmentation Analysis
To learn more about this report, Download Free Sample
Antithrombin Market Insights, By Product Type
Plasma-Derived Antithrombin owns the largest share due to its long-standing clinical validation and broad availability, proving preferred for immediate therapeutic needs. This subsegment benefits from extensive production facilities and mature supply chains, making it the backbone of current market revenue. Recombinant Antithrombin is the fastest growing segment, fueled by advances in genetic engineering that enable higher purity levels and minimized contamination risks, which is expanding its adoption, especially in developed markets with stringent safety standards.
Antithrombin Market Insights, By Application
Cardiovascular Surgery remains the leading application due to widespread usage of antithrombin in managing coagulation during procedures such as coronary artery bypass grafting and valve replacements. The increasing volume of cardiovascular surgeries globally, supported by technological advancements and rising cardiovascular disease incidence, drives stable growth in this segment. Hemodialysis holds the fastest growth, benefiting from enhanced understanding of coagulation management during dialysis and expanding dialysis patient populations worldwide.
Antithrombin Market Insights, By End-User
Hospitals account for the largest share due to the high volume of inpatient surgical procedures and critical care settings where antithrombin use is essential. Large-scale hospital networks also facilitate bulk procurement and distribution, ensuring steady market revenue. Specialty Clinics are the fastest growing subsegment, with an increasing number of outpatient anticoagulation centers and cardiac specialty units adopting antithrombin therapies for both acute management and prophylaxis.
Antithrombin Market Trends
Recent years have seen a marked uptick in the adoption of recombinant antithrombin products, propelled by favorable clinical trial outcomes demonstrating improved safety profiles and longer dosing intervals.
For instance, in 2024, a phase III trial of a novel recombinant antithrombin reported a 25% reduction in thrombotic event recurrence compared to plasma-derived products, signaling a pivotal market trend.
Another evolving trend is the increased use of digital adherence monitoring tools in home care settings, facilitating real-time therapeutic management and improved patient outcomes.
Regional diversity remains critical. North America retains market dominance with about 38% share owing to comprehensive reimbursement policies, high healthcare spending, and a vast network of plasma donation centers.
Conversely, the Asia Pacific region registers the fastest growth, driven by expanding healthcare infrastructure, increasing clinical awareness, and rising investments in biotechnology.
Antithrombin Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Antithrombin Market Analysis and Trends
In North America, the Antithrombin Market benefits from strong healthcare infrastructure, well-established plasma collection networks, and advanced biopharmaceutical manufacturing capabilities. The region’s sizable market share is supported by regulatory incentives for orphan drug development and rising cardiovascular surgery volumes, with companies such as Baxter and Grifols leading product innovation and distribution. Additionally, increased public-private collaborations have accelerated plasma supply stability, essential for maintaining market revenue streams.
Asia Pacific Antithrombin Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, driven by expanding healthcare access, increasing thrombotic disease incidence, and government policies promoting biotechnology investments. Local market players alongside multinational companies are intensifying efforts in this region, capturing emerging opportunities. The expanding patient base, coupled with rising awareness and availability of antithrombin therapies, propels market dynamics with amplified trade and export activities from manufacturing hubs in India and China.
Antithrombin Market Outlook for Key Countries
USA Antithrombin Market Analysis and Trends
The U.S. holds a pivotal role in the Antithrombin Market due to its significant cardiovascular and surgical procedure volumes, which grew 6.8% between 2023 and 2025. Regulatory frameworks such as the Orphan Drug Act have catalyzed clinical research and expedited product approvals, with Baxter and Grifols maintaining dominant market presence through innovation and distribution excellence. Furthermore, the U.S. market benefits from expansive plasma donation networks supplying key raw materials, sustaining market revenue, and competitive advantage globally.
China Antithrombin Market Analysis and Trends
China’s market is rapidly evolving with increasing recognition of congenital antithrombin deficiency cases and heightened thrombotic disease burden amid aging populations. Government initiatives to bolster biotechnology manufacturing and plasma safety standards have enhanced domestic plasma-derived antithrombin production. Leading market companies have expanded local production facilities and forged partnerships with healthcare institutions, leading to a surge in market penetration and revenue growth in this key Asia Pacific market.
Analyst Opinion
Production capacity for recombinant antithrombin has surged by approximately 15% year-over-year in 2024, reflecting increased manufacturing efficiencies and strategic expansions in biopharmaceutical facilities. This expansion directly enhances market supply dynamics, supporting the anticipated market growth rate by mitigating shortages seen in previous years.
The pricing structure is witnessing moderate volatility, with the average cost of antithrombin therapy ranging between USD 600 to USD 850 per treatment cycle in 2025, depending on formulation. Import trends reveal a strengthened demand in emerging economies, particularly in the Asia Pacific, where antithrombin imports surged by 18% in the past year due to limited domestic production capabilities.
Demand across clinical applications has diversified notably, with cardiovascular surgeries accounting for over 40% of total antithrombin consumption in 2024. The increased number of surgical procedures involving cardiopulmonary bypass notably fuels demand, supported by improved procedural success rates linked to antithrombin use.
Micro-indicators, such as increasing incidences of congenital antithrombin deficiency, estimated at 1 in 2000 to 5000 persons globally, have driven targeted R&D initiatives. This focus culminated in 2024 with approvals of next-generation antithrombin formulations offering extended half-life, contributing to enhanced patient compliance and market expansion.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.65 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.3% | 2032 Value Projection: | USD 2.78 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Grifols S.A., Baxter International Inc., Kedrion S.p.A., LFB Group, Kyowa Kirin Co., Ltd., Octapharma AG, Baxter Healthcare Corporation, CSL Behring, HTL-Strefa S.A., Takeda Pharmaceutical Company Limited. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Antithrombin Market Growth Factors
Key growth drivers in the Antithrombin Market include the rising global burden of cardiovascular and thrombotic diseases, where antithrombin therapy serves as a critical intervention; for example, cardiovascular surgeries in the U.S. increased by 7.1% between 2023 and 2025, directly elevating antithrombin demand. Technological innovations, such as advances in recombinant DNA technology, have significantly reduced production costs and improved product efficacy, boosting market growth in developed and emerging economies alike.
Additionally, favorable government policies supporting plasma collection and rare disease management have propelled market expansion, especially in regions such as North America and Europe. Increased awareness programs regarding congenital antithrombin deficiency contribute further by expanding patient diagnosis rates, enhancing market scope, and penetration.
Antithrombin Market Development
In March 2025, Sanofi received U.S. FDA approval for Qfitlia (fitusiran), the first antithrombin-lowering therapy developed for the routine prevention of bleeding episodes in adults and children with hemophilia A or B, regardless of inhibitor status. The therapy works by reducing antithrombin levels to rebalance hemostasis, offering a transformative, subcutaneous treatment option that allows for less frequent dosing compared to conventional factor replacement therapies.
In March 2025, Siemens Healthineers obtained U.S. FDA clearance for its Innovance Antithrombin assay, which serves as a companion diagnostic test for Sanofi’s Qfitlia (fitusiran). The Innovance assay enables precise monitoring of antithrombin activity levels, allowing clinicians to optimize dosing and enhance treatment safety for patients undergoing fitusiran therapy.
Key Players
Leading Companies of the Market
Grifols S.A.
Baxter International Inc.
Kedrion S.p.A.
LFB Group
Kyowa Kirin Co., Ltd.
Octapharma AG
Baxter Healthcare Corporation
CSL Behring
HTL-Strefa S.A.
Takeda Pharmaceutical Company Limited
Several leading market players have focused on strategic collaborations and acquisitions to enhance their biotechnology pipelines. For instance, Baxter International's recent acquisition of plasma fractionation facilities expanded its production capacity by 20%, strengthening its market share in North America in 2024. Meanwhile, Grifols S.A. embarked on partnerships aimed at developing recombinant antithrombin variants, boosting its product portfolio and obtaining expedited regulatory approvals in key European markets, which translated into an 8% revenue growth in 2025.
Antithrombin Market Future Outlook
Future trends in the antithrombin market point toward innovation in recombinant protein engineering, improved stabilization, and longer-acting formulations. The shift toward recombinant sources will continue as biopharmaceutical companies enhance yield efficiency and reduce production costs. Clinical research is exploring antithrombin’s potential in broader indications, including sepsis-associated coagulopathies and cardiovascular complications. The integration of antithrombin monitoring into coagulation management software is expected to enhance dosing precision in critical care. Moreover, the expansion of plasma fractionation capacity in emerging regions will improve global availability. With increasing focus on advanced hemostatic control and personalized anticoagulation therapy, antithrombin products will remain an essential component in modern clinical practice.
Antithrombin Market Historical Analysis
The antithrombin market has its origins in the 1980s with the introduction of plasma-derived concentrates to manage rare coagulation disorders. Over the years, the understanding of thrombosis and coagulation cascades has led to wider use of antithrombin in cardiovascular, obstetric, and surgical settings. Plasma-derived antithrombin products were the mainstay of therapy until recombinant technologies emerged in the 2000s, offering safer and more consistent alternatives free from blood-borne infection risks. As awareness of congenital and acquired deficiencies increased, clinical adoption expanded across intensive care and perioperative management. The past decade witnessed steady demand growth due to rising cases of disseminated intravascular coagulation and venous thromboembolism in high-risk patients.
Sources
Primary Research Interviews:
Hematologist
Clinical Pharmacologists
Biopharmaceutical Manufacturing Directors
ICU Specialists
Databases:
ClinicalTrials.gov
GlobalData Hematology Reports
WHO Blood Products Database
PubMed Therapeutic Studies
Magazines:
Pharmaceutical Technology
Biopharma Dive
Hematology Today
Drug Development News
Journals:
Blood Journal
Thrombosis and Hemostasis
Journal of Thrombosis and Thrombolysis
American Journal of Hematology
Associations:
International Society on Thrombosis and Hemostasis (ISTH)
American Society of Hematology (ASH)
World Federation of Hemophilia (WFH)
European Hematology Association (EHA)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients