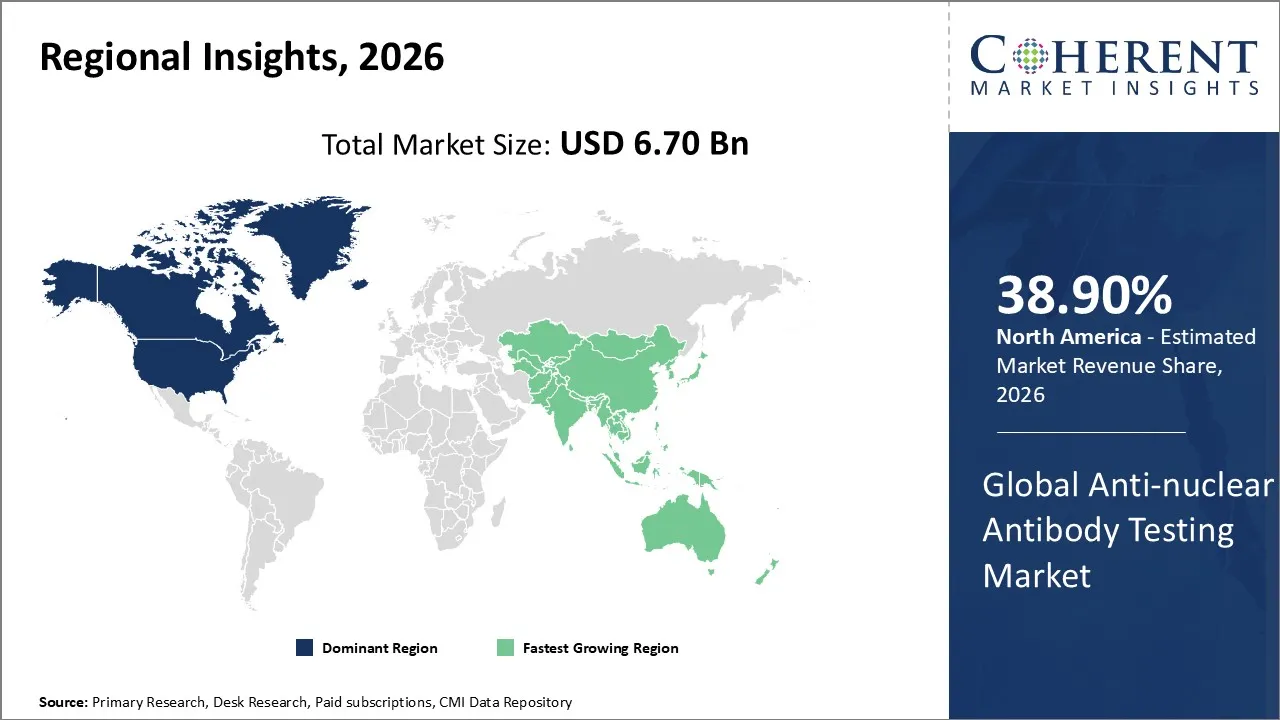
The Anti-nuclear Antibody Testing Market is estimated to be valued at USD 6.70 Bn in 2026 and is expected to reach USD 9.2 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 14.2% from 2026 to 2033.
The Anti‑nuclear Antibody (ANA) Testing Market is rapidly evolving as the in vitro diagnostics industry responds to the rising prevalence of autoimmune diseases such as lupus, rheumatoid arthritis, and Sjögren’s syndrome. Healthcare providers are increasingly aware of the importance of early diagnosis, while laboratories and hospitals adopt advanced assay technologies, including multiplex and automated platforms, to meet growing demand. Manufacturers supply reagents and assay kits as the primary consumables, with North America and Europe driving the market through well-established healthcare systems and widespread use of advanced testing methods.
|
Current Events |
Description and its impact |
|
Geopolitical Developments in Healthcare Regulations |
|
|
Technological Innovations and Integration of AI in Diagnostics |
|
|
Macroeconomic Factors Influencing Healthcare Spending |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Rheumatoid Arthritis expected to hold largest market share of 34.6% in 2026. Rheumatoid arthritis drives the Anti‑nuclear Antibody (ANA) Testing Market as clinicians rely on ANA tests to diagnose and monitor disease progression. Because RA patients often exhibit overlapping autoimmune symptoms, healthcare providers perform broader antibody screenings to differentiate it from conditions like lupus and Sjögren’s syndrome. Emphasizing early detection and personalized treatment, hospitals and laboratories conduct regular testing, while advanced assay technologies deliver faster and more accurate results, reinforcing the widespread adoption of ANA tests in managing rheumatoid arthritis. For instance, in July 2025, Agilus Diagnostics, in partnership with Sebia, has launched the anti-MCV antibody test in India to support early rheumatoid arthritis diagnosis, especially in patients negative for anti-CCP and rheumatoid factor.
Reagents & Assay Kits hold the largest market share of 24.3% in 2026. Reagents and assay kits drive the Anti‑nuclear Antibody (ANA) Testing Market as laboratories and hospitals depend on these consumables for accurate and efficient autoimmune disease diagnostics. Clinics use ready-to-use kits and high-quality reagents to test conditions like lupus and rheumatoid arthritis effectively. Advances in multiplex assays and automated platforms improve test reliability and streamline workflows, while the continuous demand for consumables in routine screening and patient monitoring sustains steady growth throughout clinical and diagnostic environments. For instance, in November 2024, Becton, Dickinson, and Company (BD) announced the commercial launch of the BD OMICS-One XT WTA Assay, its first robotics-compatible, high-throughput reagent kit.
Multiplex Assay acquired the prominent market share of 37.7% in 2026. Multiplex assays drive growth in the Anti‑nuclear Antibody (ANA) Testing Market by allowing laboratories to detect multiple autoantibodies from a single sample, saving time and resources. Hospitals and clinics adopt these assays to streamline workflows, minimize sample requirements, and enhance diagnostic accuracy for complex autoimmune diseases. By integrating with automated, high-throughput platforms, labs boost efficiency, while clinicians use the comprehensive results to make faster, informed decisions. The combination of speed, accuracy, and convenience is increasing the preference for multiplex assays in ANA testing. For instance, in July 2025, Leica Biosystems unveiled ChromoPlex III Triple Detection RUO, a chromogenic multiplex immunohistochemistry system designed to simplify assay setup and minimize variability.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 38.90% in 2026. North America drives the Anti‑nuclear Antibody (ANA) Testing Market through its advanced healthcare infrastructure and strong adoption of innovative diagnostic technologies. Hospitals and reference laboratories implement automated and multiplex platforms to increase testing speed and accuracy. Clinicians’ growing awareness of autoimmune diseases, along with well-established reimbursement systems, encourages routine ANA screening. Major diagnostic companies and ongoing research initiatives continuously develop new products, shaping market trends that focus on efficiency, accuracy, and broader access to ANA testing throughout the region. For instance, Organon & Co. and Samsung Bioepis have launched HADLIMA™ (adalimumab-bwwd), a Humira biosimilar, in the U.S., offering both high- and low-concentration options for seamless patient care.
The Asia Pacific region is expanding the Anti‑nuclear Antibody (ANA) Testing Market by improving healthcare infrastructure and increasing awareness of autoimmune diseases. Hospitals and diagnostic laboratories adopt modern assay technologies, including automated and multiplex platforms, to boost testing efficiency and accuracy. Governments implement initiatives, rising healthcare spending supports advanced diagnostics, and greater access encourages routine ANA screening. Local manufacturers and international collaborations increase product availability, actively driving the region toward more comprehensive and timely autoimmune testing. In July 2025, Agilus Diagnostics, with Sebia, launched the Anti-MCV antibody test in India to improve early rheumatoid arthritis detection, particularly in patients negative for anti-CCP and rheumatoid factor.
The United States drives the Anti‑nuclear Antibody (ANA) Testing Market through its well-established healthcare system and widespread use of advanced diagnostic technologies. Hospitals and clinical laboratories adopt automated and multiplex platforms to improve testing accuracy and reduce turnaround times. Physicians’ growing awareness of autoimmune diseases, supported by insurance coverage and reimbursement policies, encourages routine ANA screening. Leading diagnostic companies conduct active research and development, introducing innovative tests, expanding availability, and reinforcing the country’s role as a central hub for comprehensive autoimmune diagnostics.
China is actively expanding the Anti‑nuclear Antibody (ANA) Testing Market by modernizing healthcare infrastructure and increasing awareness of autoimmune diseases. Hospitals and diagnostic laboratories adopt automated and multiplex assay technologies to boost testing speed, accuracy, and efficiency. Government initiatives and rising healthcare investment broaden access to diagnostic services, encouraging routine ANA screening. Local manufacturers collaborate with international diagnostic companies to increase product availability and drive innovation, positioning the country to deliver more comprehensive, timely, and reliable autoimmune disease diagnostics across both urban and emerging areas.
The ANA testing market is moving toward multiplex and high-throughput platforms, allowing simultaneous detection of multiple autoantibodies from a single sample. Laboratories adopt these technologies to increase efficiency, reduce sample volume, and minimize turnaround times. Clinicians benefit from comprehensive profiles that support accurate diagnosis of complex autoimmune disorders. This trend reflects the growing demand for faster, reliable, and integrated testing solutions that streamline workflows while providing detailed immunological insights for personalized patient care.
Automation and digital integration are reshaping ANA testing practices. Laboratories implement automated immunoassay analyzers and AI-assisted interpretation tools to enhance precision and reduce human error. Digital platforms facilitate result management, data tracking, and integration with hospital information systems. This trend addresses clinician and technician demands for faster, reproducible, and standardized results, supporting efficient workflow management and consistent clinical decision-making across hospitals, reference laboratories, and specialized diagnostic centers.
There is a strong opportunity to integrate ANA testing with automated analyzers and digital reporting systems. This allows laboratories to streamline workflows, reduce manual errors, and provide clinicians with faster, more reliable results. Manufacturers can develop assay kits compatible with high-throughput and AI-assisted platforms, catering to hospitals and large diagnostic networks seeking standardized, scalable, and technologically advanced testing solutions, reinforcing efficiency and clinical accuracy.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 6.70 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 14.2% | 2033 Value Projection: | USD 9.2 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
F. Hoffmann-La Roche Ltd., ERBA Diagnostics, Zeus Scientific, Inc., Alere Inc., Bio-Rad Laboratories, Inc., Becton Dickinson and Company, Antibodies, Inc., EUROIMMUN AG, Immuno Concepts, Inova Diagnostics, Thermo Fisher Scientific, Inc., and Trinity Biotech plc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients