Global Anti-inflammatory drugs market is estimated to be valued at USD 115.55 Bn in 2025 and is expected to reach USD 210.02 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.9% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Rising geriatric population suffering from arthritis and other joint disorders can boost demand for anti-inflammatory drugs for pain management. Increasing adoption of analgesic drugs due to changing lifestyle patterns and rising awareness regarding treatment of chronic conditions can drive the market growth. However, side effects associated with long term use of certain anti-inflammatory drugs along with availability of alternative treatment options can hamper the market growth in the near future. Introduction of advanced drugs with lesser side effect profile can offer market growth opportunities.
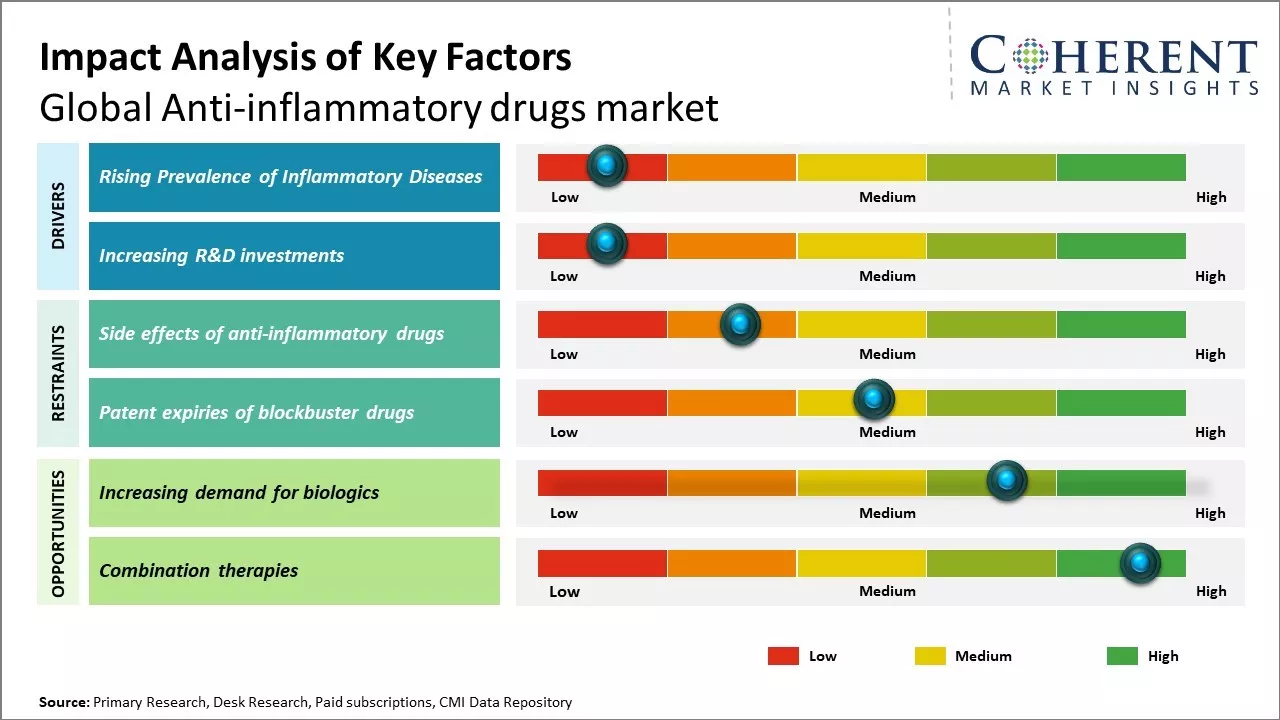
Rising Prevalence of Inflammatory Diseases
Increasing incidence and prevalence of various inflammatory diseases across the globe can drive the anti-inflammatory drugs market growth. Inflammation is the body's natural response to injuries or infections, however, chronic or long-term inflammation can damage tissues and even cause other diseases. Rising sedentary lifestyle and changing dietary patterns have made people more prone to obesity, diabetes and cardiovascular issues all of, which have an underlying inflammatory component. According to various medical studies, obesity, which has become a global epidemic, has direct links to inflammation in the body. Adipose or fat tissue when accumulated in large quantities triggers the immune system to produce abnormal levels of cytokines, which are messenger molecules that cause chronic inflammation. High blood sugar levels seen in diabetes patients lead to increased oxidative stress and free radical formation, damaging cells and tissues over time. Other diseases like atherosclerosis, rheumatoid arthritis, multiple sclerosis also manifest due to prolonged inflammation. For instance, according to an article published by MDPI Journal, the incidence of inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), has been increasing globally including Asia Pacific. Previously more common in Western countries, IBD now shows significant prevalence in Asian populations due to changing lifestyles, diets, and environmental factors. IBD is increasingly prevalent due to changing lifestyles and environmental factors. In Asia, UC has a single peak age at diagnosis, while CD shows two peaks, differing from the West. Interestingly, males in Asia have higher IBD rates compared to females, unlike in the West. Genetic factors play a significant role, with variations seen between Caucasian and non-Caucasian populations. Environmental factors like smoking and hygiene practices can also led to development of inflammatory bowel disease.
Get actionable strategies to beat competition: Download Free Sample
Increasing R&D investments
Increasing research and development investments by pharmaceutical companies can drive the global anti-inflammatory drugs market growth. These companies are investing heavily in R&D activities to develop novel and more effective treatment options for inflammation-related chronic diseases. According to the data from Organisation for Economic Co-operation and Development (OECD), the average R&D spending as a percentage of sales by pharmaceutical firms had increased from 12.3% in 2010 to 13.4% in 2020. The existing anti-inflammatory drugs are associated with several side effects on long-term use such as gastrointestinal issues. Therefore, there is high need for advanced anti-inflammatory treatment with improved safety profiles. The R&D efforts are primarily focused on developing biologics and new targeted therapies to treat chronic inflammation. For example, companies are researching on cytokine and chemokine pathway inhibitors that can block specific molecules responsible for inflammation. Some firms are also exploring stem cell and gene therapies for rheumatoid arthritis and inflammatory bowel disease.
Key Takeaways from Analyst:
Rising incidence of inflammation-related conditions such as arthritis can boost demand for anti-inflammatory drugs. Growth in the osteoarthritis drug segment can drive the market growth. Increasing awareness about arthritis management can boost usage of these drugs. While developed nations account for the majority of the current market, developing countries are likely to emerge as lucrative opportunities due to growing aging populations and access to healthcare.
Side effects of long-term use of NSAIDS and high development and regulation costs for novel drugs can hamper the market growth. Biosimilars may capture a portion of the market. However, introduction of combination therapies and pipeline drugs with improved safety profiles can offer market growth opportunities. The use of AI and big data in clinical trials can help streamline drug development and reduce costs.
North America currently dominates the market due to high healthcare spending and rising arthritis burden. Asia Pacific is forecast to be the fastest growing regional market. China and India's massive populations, improving standards of living and expanding health insurance can drive the market growth. Generics penetration will continue lifting volumes in cost-sensitive developing markets.
Market Challenges: Side effects of anti-inflammatory drugs
The side effects of anti-inflammatory drugs can hamper the global anti-inflammatory drugs market growth. While anti-inflammatory drugs are effective in providing relief from conditions like arthritis, back pain and general aches and pains, these can also often cause undesirable and dangerous side effects. Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen are among the most commonly used anti-inflammatory medicines. However, their prolonged use over months and years have been associated with severe issues like gastrointestinal problems and kidney damage. Long term NSAID users have reported ulcers, holes in the stomach or intestine and in rare cases even stomach or intestinal bleeding. This can especially be a problem for elderly patients managing arthritis. In 2022, according to the study published by American Journal of Gastroenterology, over 30,000 Americans are hospitalized each year due to NSAID-induced gastrointestinal toxicity, resulting in thousands of deaths. Corticosteroids are another class of powerful anti-inflammatory medicines prescribed for conditions like rheumatoid arthritis and lupus. While effective in reducing joint swelling and pain from autoimmune diseases, long term or frequent use can have serious implications
Market Opportunities: Increasing demand for biologics
Global anti-inflammatory drugs market has witnessed huge demand and interest for biologics in the recent years. Biologics, also known as biological drugs, are made from living cells and have revolutionized treatments for chronic inflammatory diseases. Compared to traditional small molecule anti-inflammatory drugs, biologics provide highly targeted therapies with greater effectiveness and less systemic side effects. Leading health organizations report that immune-mediated inflammatory diseases like arthritis, bowel diseases and psoriasis are rising globally. According to the World Health Organization, over 300 million people worldwide suffer from rheumatoid arthritis and osteoarthritis. Due to growing aging population and changes in lifestyle, there has been rising prevalence of autoimmune diseases. This rising disease burden coupled with limitations of conventional drugs has accelerated research and development of new biologic treatments.
Biologics have already achieved major breakthroughs in treating inflammatory conditions that were previously difficult to manage such as Crohn's disease, ulcerative colitis and psoriatic arthritis. Advanced biologics are more tailored towards specific disease pathways with innovative drug delivery mechanisms and biosimilars are also expanding access at reduced costs compared to originators. As biologics continue demonstrating significant clinical efficacy over long term use, more pharmaceutical developers are investing heavily in this area of drug development.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
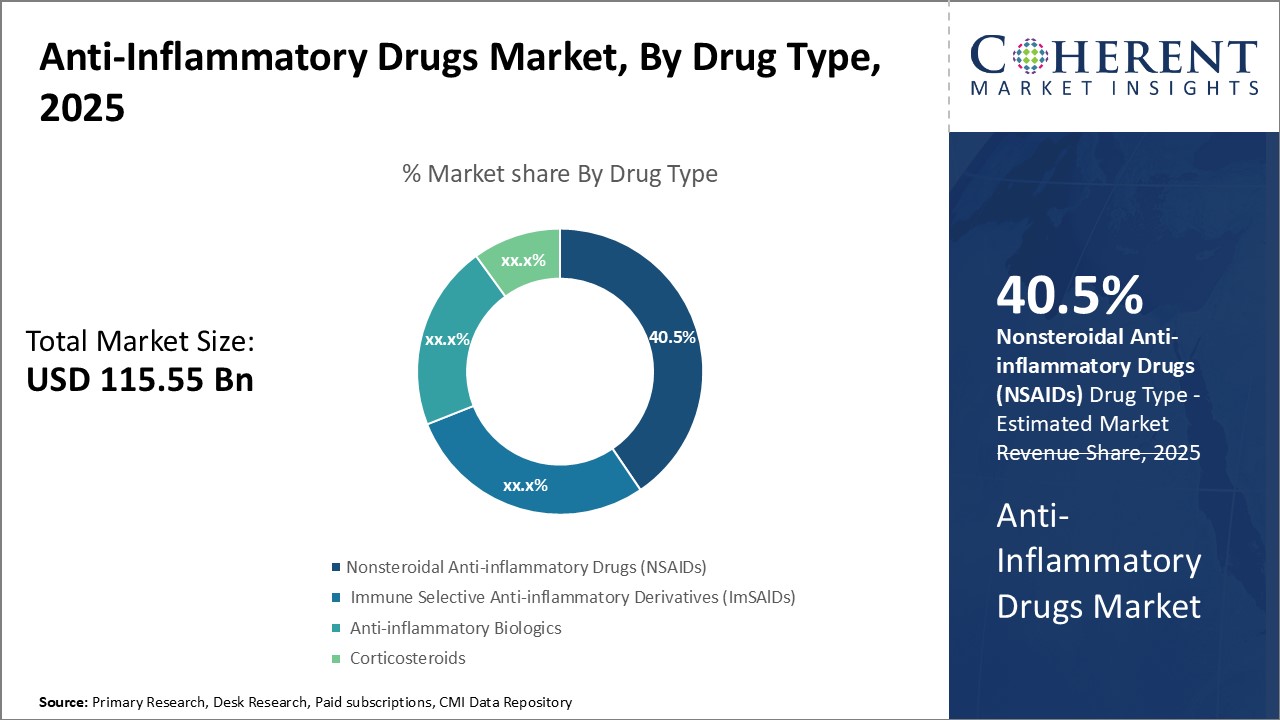
By Drug Type- Easy availability drives highest market share of nonsteroidal anti-inflammatory drugs
In terms of drug type, nonsteroidal anti-inflammatory drugs (NSAIDs) segment is estimated to contribute the highest market share of 40.5% in 2025, owing to their easy over-the-counter availability and long history of usage. NSAIDs are the most common class of medications that are prescribed for relief of pain, fever, and inflammation. Unlike other anti-inflammatory drugs, NSAIDs can be easily purchased without a prescription from pharmacies and retail stores for temporary relief from minor bodily aches and pains. As these have been in use for several decades, patients as well as physicians are quite familiar with generic NSAID medications like aspirin, ibuprofen and naproxen. Their widespread non-prescription access aids self-medication and enhances patient compliance for regular usage.
By Application- Frequent diagnosis sustains dominance of arthritis
In terms of application, arthritis segment is estimated to contribute the highest market share of 20.5% in 2025, owing to its high prevalence and frequent diagnosis rates. Arthritis is a common condition characterized by joint pain and stiffness, with osteoarthritis and rheumatoid arthritis being the most common types. As per studies, arthritis affects over 300 million people worldwide with the prevalence steadily growing due to an aging population and rising obesity levels. The condition requires long-term or lifetime management, thus, increasing reliance on anti-inflammatory drugs for pain relief. The scale of arthritis diagnosis and treatment exerts a major influence on the global anti-inflammatory drugs market.
By Route of Administration- Ease of oral administration amplifies oral route dominance
In terms of route of administration, oral segment is estimated to contribute the highest market share of 25.5% in 2025, due to ease and convenience of oral drug delivery. The oral route is the most natural and comfortable method of drug administration for majority of anti-inflammatory medication. Oral anti-inflammatory tablets and capsules are easy to consume, prepped in convenient unit dosages and involve minimal technique or training. This simplicity has made the oral route preferable over injected or inhaled alternatives from both patient and healthcare provider perspective. The undemanding nature of oral drug administration amplifies patient adherence and boosts its widespread use over other delivery modes.
Need a Different Region or Segment? Download Free Sample
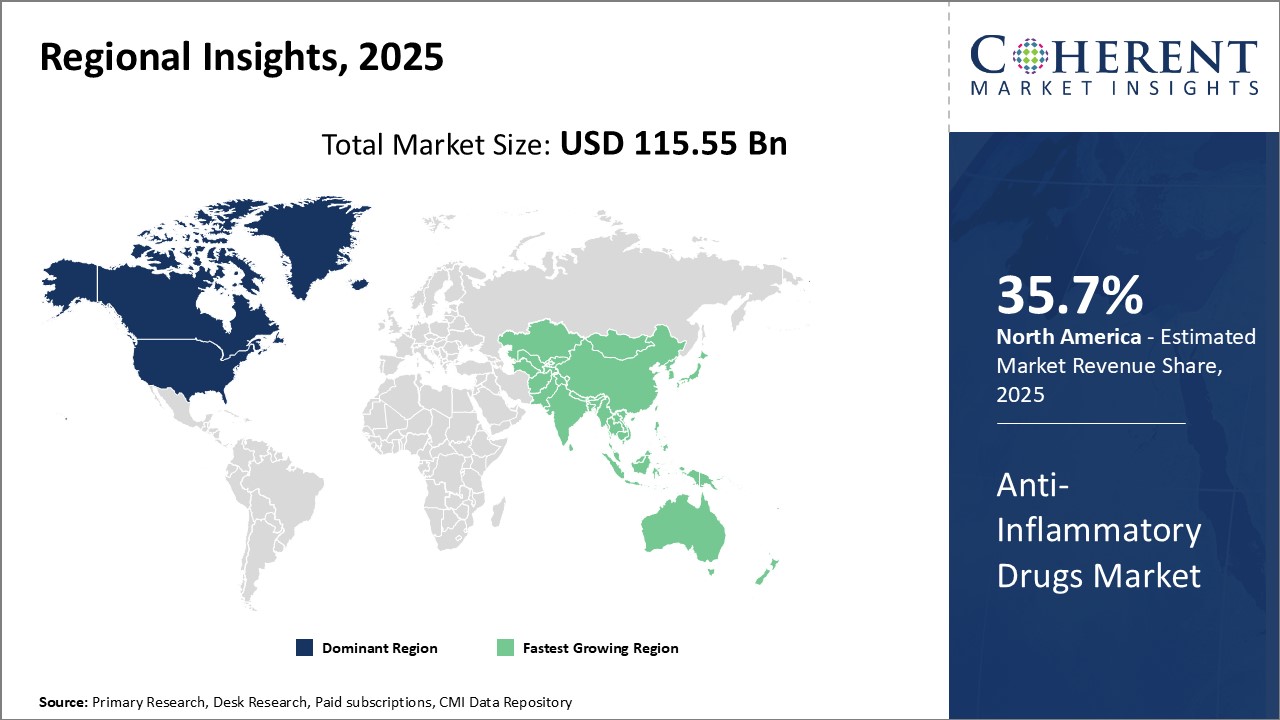
North America dominates the global anti-inflammatory drugs market with an estimated market share of 35.7% in 2025. With presence of leading pharmaceutical companies based in countries like the U.S. and Canada, the region accounts for the largest share of research and development activities. Many new drug formulations are launched and approved in the region first before being made available in other parts of the world. Furthermore, countries in North America have well-established healthcare systems that provide affordable access to innovative drugs, thus, boosting higher consumption. The on-going developments in biosimilars by major players enable cost savings, which can increase patients pool undergoing treatment over the coming years.
Asia Pacific emerges as fastest-growing market for anti-inflammatory drugs. Several economic and healthcare industry have contributed to the rapid expansion.. Countries like India, China, South Korea, and Japan are prioritizing healthcare spending to address the rising incidence of chronic diseases. This has encouraged both domestic and multinational companies to expand their presence through manufacturing and distribution networks across Asia Pacific. Rising disposable incomes allows more patients in the middle-income Asian countries to opt for branded anti-inflammatory drugs over cheap generics. While import dependence remains high currently, local drug makers are investing aggressively into R&D to develop complex anti-inflammatory drugs indigenously.
Anti-inflammatory Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 115.55 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.9% | 2032 Value Projection: | USD 210.02 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
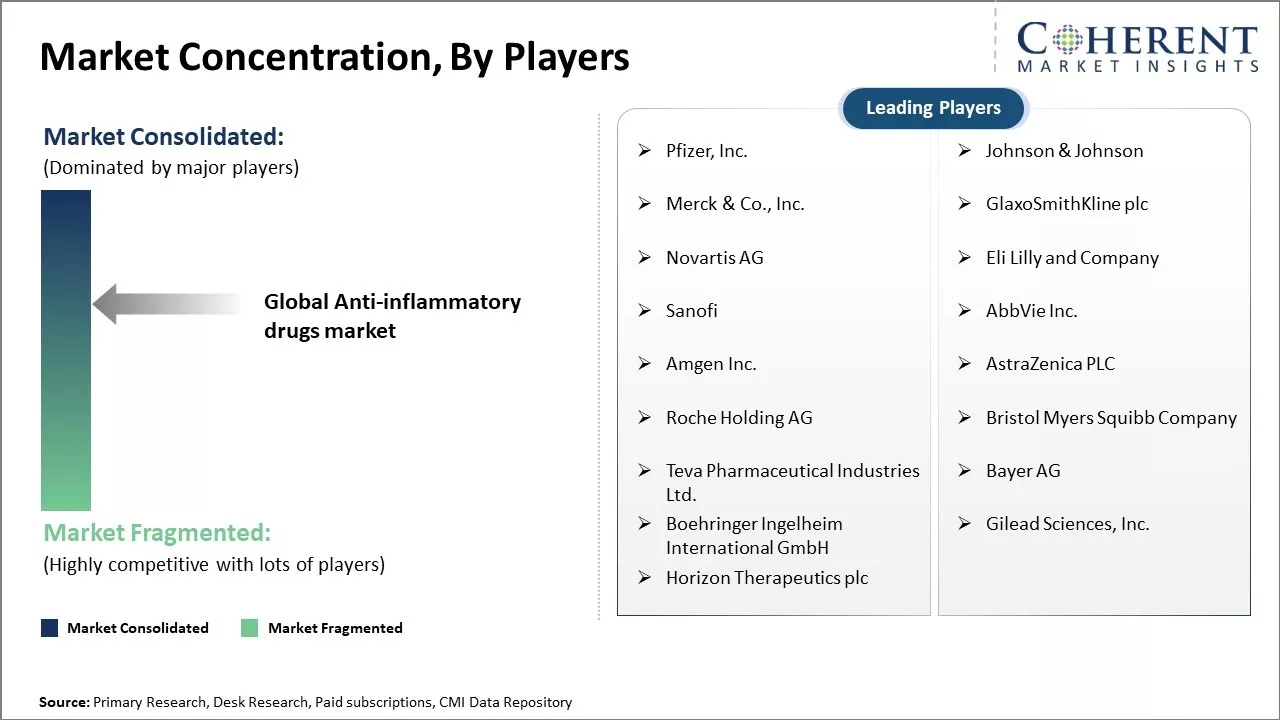
Pfizer, Inc., Johnson & Johnson, Merck & Co., Inc., GlaxoSmithKline plc, Novartis AG, Eli Lilly and Company, Sanofi, AbbVie Inc., Amgen Inc., AstraZeneca PLC, Roche Holding AG, Bristol Myers Squibb Company, Teva Pharmaceutical Industries Ltd., Bayer AG, Boehringer Ingelheim International GmbH, Gilead Sciences, Inc., Horizon Therapeutics plc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global Anti-inflammatory drugs market consists of pharmaceutical products used for reducing pain, fever, stiffness, and swelling caused by inflammation or injury. It includes non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, and aspirin, as well as, corticosteroids such as prednisone and dexamethasone. The market covers prescription and over-the-counter drugs used for relieving symptoms caused by conditions like arthritis, injuries, infections, and other inflammatory disorders worldwide. The demand is driven by rising geriatric population and growing prevalence of inflammatory and pain-related diseases globally.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients