Anti-infective Drugs Market Size and Forecast – 2025 – 2032
The Global Anti-infective Drugs Market size is estimated to be valued at USD 105.8 billion in 2025 and is expected to reach USD 160.3 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.3% from 2025 to 2032.
Global Anti-infective Drugs Market Overview
Anti-infective drugs include a broad spectrum of antibacterial, antiviral, antifungal, and antiparasitic agents designed to prevent or treat infections caused by pathogens. Core product categories consist of broad-spectrum antibiotics, antivirals for influenza, HIV, and hepatitis, antifungal agents like azoles and echinocandins, and antimalarial compounds. However, rising antimicrobial resistance (AMR) has spurred innovation in novel drug classes, combination therapies, and bacteriophage-based treatments. The introduction of long-acting formulations, host-directed therapies, and nanoparticle-based delivery systems has improved efficacy and patient compliance.
Key Takeaways
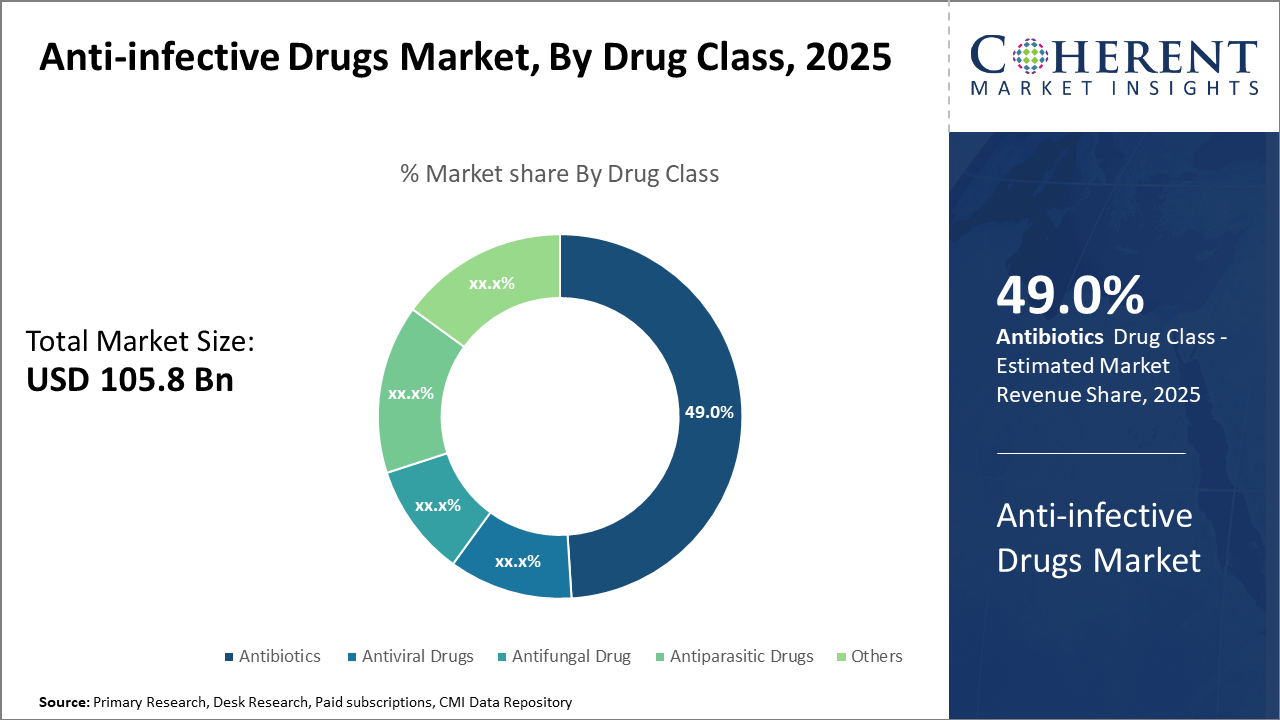
The antibiotics segment retains dominance with nearly 49% of the market share, driven by steady demand and innovation in beta-lactam and macrolide classes. Notably, the antiviral drugs segment is rapidly growing due to the rising incidence of viral infections and expanded prophylactic use, reflecting evolving market trends.
Parenteral route surpasses others with a 57% share, particularly for hospital-acquired infections where rapid administration is critical, while oral administration remains steady due to outpatient treatment preferences.
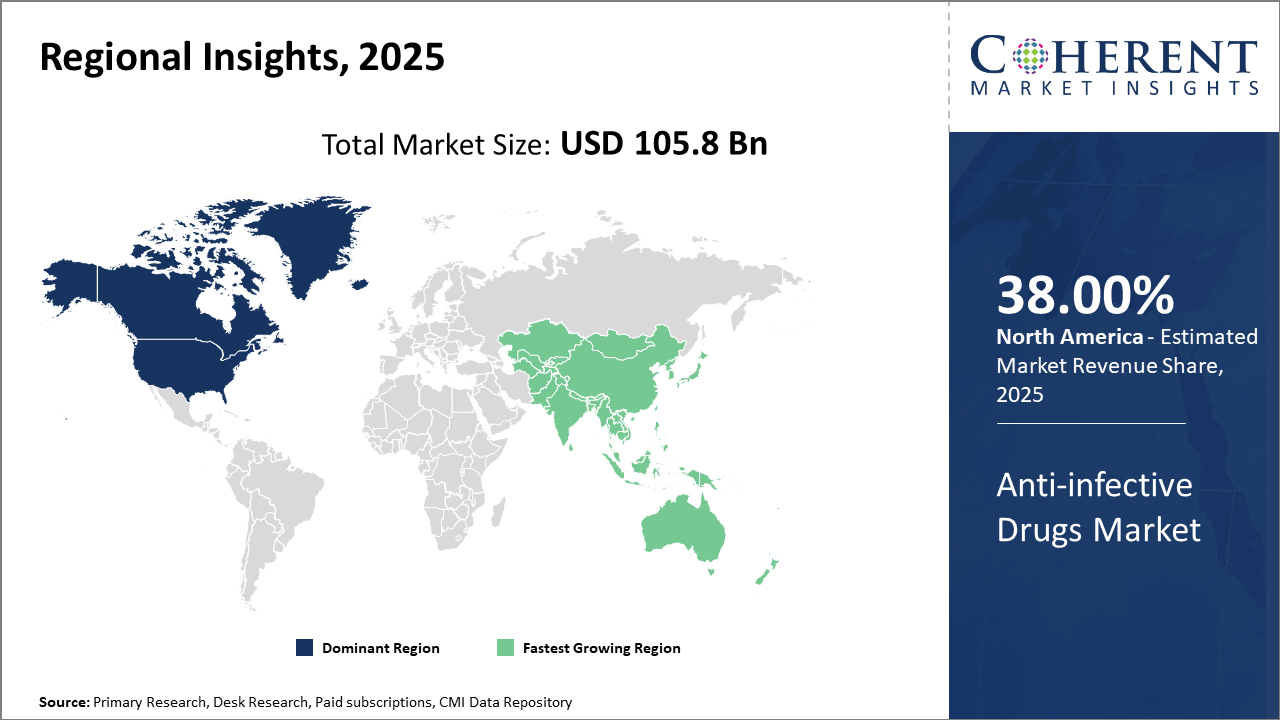
North America sustains its dominance in the Anti-infective Drugs Market with over 38% industry share, primarily attributed to robust R&D infrastructure and proactive health policies.
Meanwhile, Asia Pacific emerges as the fastest-growing region, registering a CAGR exceeding 8%, benefiting from expanding healthcare access and increasing infectious disease burden.
Anti-infective Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
Anti-infective Drugs Market Insights, By Drug Class
Antibiotics dominate the market share with 49%. The dominance of antibiotics stems from their broad therapeutic applicability across diverse infections, including respiratory, urinary tract, and skin infections. Advanced classes such as beta-lactams remain fundamental in treatment regimens, while emerging macrolides and fluoroquinolones are expanding due to resistance management. The fastest-growing subsegment is Antiviral Drugs, primarily propelled by increased viral outbreaks such as novel influenza strains and emerging coronaviruses. Investments in antiviral therapeutics have substantially increased, particularly for prophylaxis in immunocompromised patients.
Anti-infective Drugs Market Insights, By Route of Administration
Parenteral segment is the dominating subsegment, holding 57% market share. Parenteral administration is favored especially in hospital-acquired infections and severe systemic infections, where rapid and controlled drug delivery is critical for efficacious outcomes. Intravenous and intramuscular injections allow for high bioavailability, ensuring fast therapeutic action. Fastest growth is observed in the Oral segment, attributable to patient convenience and outpatient treatment trends encouraging oral anti-infective regimens. Recent formulation advancements have improved oral bioavailability for several drugs traditionally limited to parenteral routes.
Anti-infective Drugs Market Insights, By Application
The Community-Acquired Infections segment leads in market share. This dominance reflects the wide prevalence of respiratory and urinary infections treated predominantly in outpatient settings, driving consistent market demand. Major focus on outpatient antibiotic stewardship programs further supports sustained growth in this segment. Hospital-acquired infections represent the fastest-growing subsegment, primarily due to the increasing incidence of multidrug-resistant pathogens in healthcare facilities. The critical nature of such infections demands advanced drug formulations and parenteral delivery routes, pushing market revenue upward. Chronic Infectious Diseases, including HIV/AIDS and tuberculosis, retain a stable market presence due to ongoing requirements for long-term care and prophylaxis.
Anti-infective Drugs Market Trends
The Anti-infective Drugs market trend strongly emphasizes the integration of digital tools and personalized medicine.
For instance, the adoption of AI-assisted diagnostic platforms in 2025 has accelerated the identification of resistant pathogens, enabling more precise drug prescriptions and reducing unnecessary antibiotic use.
Moreover, the shift towards biologics like monoclonal antibodies is setting new therapeutic benchmarks, as seen in the 2024 approval of a novel antibody treatment for viral infections with unprecedented efficacy.
Another critical trend involves public health-driven expansion of prophylactic drug administration, driven by successful programs in malaria-endemic regions.
Anti-infective Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Anti-infective Drugs Market Analysis and Trends
In North America, the dominance in the Anti-infective Drugs market is driven by advanced healthcare infrastructures and substantial investments in antimicrobial resistance research. The U.S. accounts for the majority of this region’s market share, bolstered by government incentives and robust pharma R&D activity. Companies such as Pfizer and Merck actively contribute through pipeline diversification and stewardship initiatives, reinforcing the region's leadership in market revenue and innovation.
Asia Pacific Anti-infective Drugs Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, propelled by expanding healthcare access, rising infectious disease incidence, and growing pharmaceutical manufacturing capabilities. Governments in India and China are intensifying spending on infectious disease control, which supports escalating demand for anti-infective drugs. Companies like Cipla and Sun Pharmaceutical play pivotal roles in regional business growth, leveraging cost advantages and local distribution networks.
Anti-infective Drugs Market Outlook for Key Countries
USA Anti-infective Drugs Market Analysis and Trends
The U.S. market remains a significant hub for anti-infective drug innovation and commercial activity, accounting for approximately 30% of the global market revenue. The FDA’s accelerated approval pathways have facilitated the launch of multiple novel agents targeting resistant bacterial and viral strains in 2024 and 2025. Pharmaceutical behemoths headquartered in the U.S., such as Johnson & Johnson and Gilead Sciences, are focusing on expanding their biologics portfolios and developing next-generation oral therapies. Public health initiatives aimed at reducing hospital-acquired infection rates bolster demand for advanced parenteral formulations, ensuring continued market dominance.
India Anti-Infective Drugs Market Analysis and Trends
India’s rising population and persistent infectious disease burden position it as a rapidly evolving market, with increasing governmental support for disease eradication programs, including tuberculosis and malaria. Indian pharma giants like Cipla and Sun Pharmaceutical have ramped up production capacities to meet both domestic demand and international exports, contributing significantly to market growth. The prevalence of antimicrobial resistance has also driven innovation in novel generics and biosimilars, alongside increasing penetration of combination therapies. Furthermore, expanding healthcare infrastructure and rising disposable incomes support the broader adoption of anti-infective drugs.
Analyst Opinion
Innovations in Drug Formulation and Delivery: Recent advancements in nano-formulations and sustained-release drug delivery systems have enhanced the efficacy and patient compliance of anti-infective drugs. For example, in 2024, a leading pharmaceutical firm successfully launched a liposomal formulation for antifungal agents, which demonstrated a 20% reduction in dosing frequency, positively impacting market uptake.
Rising Incidence of Drug-Resistant Pathogens: The accelerated spread of multidrug-resistant organisms (MDROs) significantly fuels the demand for novel anti-infective drugs, particularly in hospital settings. WHO reports in 2025 reveal a 15% increase in antimicrobial resistance cases globally compared to 2023, validating this critical market driver.
Expansion of Preventive Chemotherapy and Prophylaxis: Growth in preventive healthcare initiatives, especially in endemic regions affected by tuberculosis and malaria, has increased the consumption of anti-infective drugs for prophylactic use. Data from the Global Fund in early 2025 indicate a 12% year-over-year rise in procurement of such drugs in sub-Saharan Africa alone.
Growing Investments in Biologics and Monoclonal Antibodies: The surge in research funding towards biologic anti-infectives, including monoclonal antibodies targeting viral infections, reflects a transformative shift in treatment paradigms. Recent clinical trials in 2024 showcased a monoclonal antibody with 85% efficacy against a novel virus strain, accelerating market growth in this niche.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 105.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.3% | 2032 Value Projection: |
USD 160.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., GlaxoSmithKline plc, Johnson & Johnson, Merck & Co., Inc., Novartis AG, Roche Holding AG, Sanofi S.A., AstraZeneca plc, Bayer AG, F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company, Astellas Pharma Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Anti-infective Drugs Market Growth Factors
The rising burden of infectious diseases worldwide remains the foremost driver for the anti-infective drugs market growth, with the WHO highlighting a 10% surge in global infectious outbreaks between 2023 and 2025. Technological innovations in drug development, particularly the integration of AI-driven platforms for rapid molecule screening, have shortened new drug approval timelines, underpinning market revenue expansion. Increasing government initiatives and funding for antimicrobial stewardship programs, especially in North America and Europe, have further stimulated growth by enhancing treatment protocols and expanding usage. Additionally, an expanding geriatric population globally, prone to infections and co-morbidities, fuels sustained demand for effective anti-infective therapies.
Anti-infective Drugs Market Development
In September 2025, Cipla Limited launched HUENA® (methenamine hippurate), marking India’s first non-antibiotic drug for the prevention of recurrent urinary tract infections (UTIs). The treatment offers an antimicrobial-resistance-friendly alternative to long-term low-dose antibiotics.
In July 2025, Lupin Limited received approval from the U.S. Food & Drug Administration (FDA) for two complex generics – liraglutide injection (single-patient-use prefilled pen) and glucagon for injection (vial) – manufactured at its injectable facility in Nagpur, India. The company is set to launch these products in the U.S. market.
Key Players
Leading Companies of the Market
Pfizer Inc.
GlaxoSmithKline plc
Johnson & Johnson
Merck & Co., Inc.
Novartis AG
Roche Holding AG
Sanofi S.A.
AstraZeneca plc
Bayer AG
F. Hoffmann-La Roche Ltd
Bristol-Myers Squibb Company
Astellas Pharma Inc.
Several leading companies have adopted aggressive growth strategies to retain and expand their market shares. For instance, Pfizer’s strategic acquisition of a biotech firm specializing in novel antiviral molecules in late 2024 significantly enhanced its product pipeline, resulting in a 10% boost in market revenue by Q1 2025. Similarly, GlaxoSmithKline has leveraged public-private partnerships to strengthen its foothold in emerging markets, which contributed to a 15% increase in regional market share over the past year. These competitive strategies highlight the dynamic business growth approaches shaping the current industry landscape.
Anti-infective Drugs Market Future Outlook
The future of the anti-infective market will be shaped by a dual imperative: accelerating innovation while ensuring sustainable commercial models. Scientific advances—in phage therapy, antibody therapeutics, small molecules with novel mechanisms, and host-directed therapies—offer promising leads, but scaling and commercialization will rely on creative reimbursement (subscription/pull) models and government procurement strategies. Rapid point-of-care diagnostics will be critical to enable targeted use of novel agents and to limit AMR selection pressure. Global collaboration, increased funding for early-stage discovery, and clearer regulatory pathways for narrow-spectrum agents will be essential. In parallel, stewardship programs, diagnostics integration, and vaccines will reduce infection incidence, reshaping demand dynamics
Anti-infective Drugs Market Historical Analysis
Anti-infective therapeutics have been central to medicine since the antibiotic era began with penicillin, but their effectiveness has been increasingly challenged by antimicrobial resistance (AMR). For decades, pharmaceutical innovation in antibiotics slowed due to poor commercial returns and scientific difficulty; meanwhile, antivirals and antifungals advanced in targeted niches (HIV, HCV, systemic fungal diseases). The 2000s and 2010s saw renewed policy attention and incentives—push/pull mechanisms, priority review vouchers, and PRV-like incentives—intended to revive antibiotic R&D. Simultaneously, diagnostics and stewardship programs sought to preserve existing agents while enabling targeted therapy. Recent years have produced a few novel agents against resistant Gram-negative bacteria and improved antiviral classes, but the pipeline remains fragile and highly dependent on public-private partnerships and alternative commercial models.
Sources
Primary Research Interviews:
Infectious Disease Specialists
Microbiologists
Clinical Pharmacologists
Public Health Experts
Databases:
WHO Global Health Observatory
CDC Infectious Disease Data
PubChem
ClinicalTrials.gov
Magazines:
Pharmaceutical Executive
Nature Medicine
The Pharma Letter,
Infection Control Today
Journals:
Clinical Infectious Diseases
The Lancet Infectious Diseases
Emerging Infectious Diseases
Associations:
WHO
Infectious Diseases Society of America (IDSA)
European Society of Clinical Microbiology (ESCMID)
CDC
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients