Angioedema Treatment Market Size and Forecast – 2025 – 2032
The Global Angioedema Treatment Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.3 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.4% from 2025 to 2032.
Global Angioedema Treatment Market Overview
Angioedema treatment products are designed to manage acute and chronic episodes of localized swelling caused by allergic reactions, hereditary deficiencies, or medication side effects. The therapeutic range includes antihistamines, corticosteroids, C1 esterase inhibitor concentrates, bradykinin receptor antagonists, and monoclonal antibodies. Treatments are available in oral, injectable, and intravenous forms depending on severity and underlying cause. Advances in biologics have led to the development of targeted therapies for hereditary angioedema (HAE), improving disease control and reducing attack frequency.
Key Takeaways
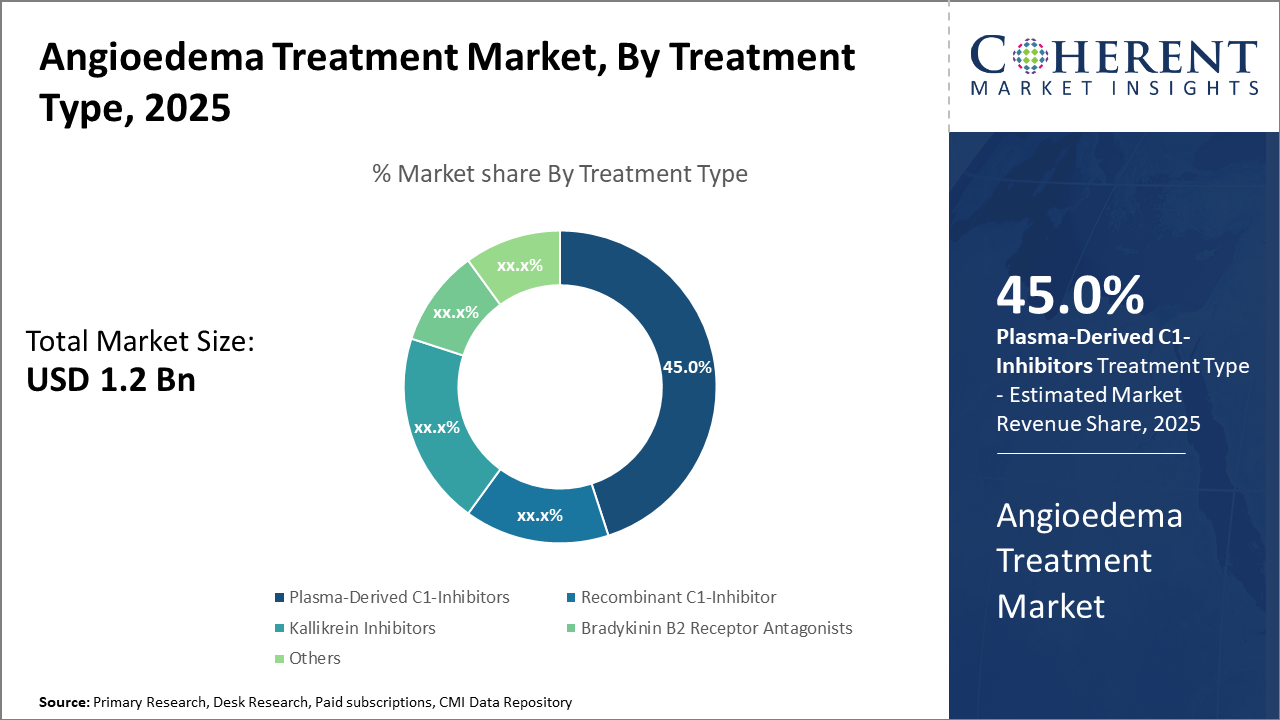
The Plasma-Derived C1-Inhibitors segment commands an industry size with approximately 45% market share, driven by strong clinical efficacy and established reimbursement frameworks.
Meanwhile, oral kallikrein inhibitors represent the fastest-growing treatment type due to ease of administration and expanding indications.
The hereditary angioedema subsegment accounts for the largest market share within disease types, reflecting its higher prevalence and diagnostic rates.
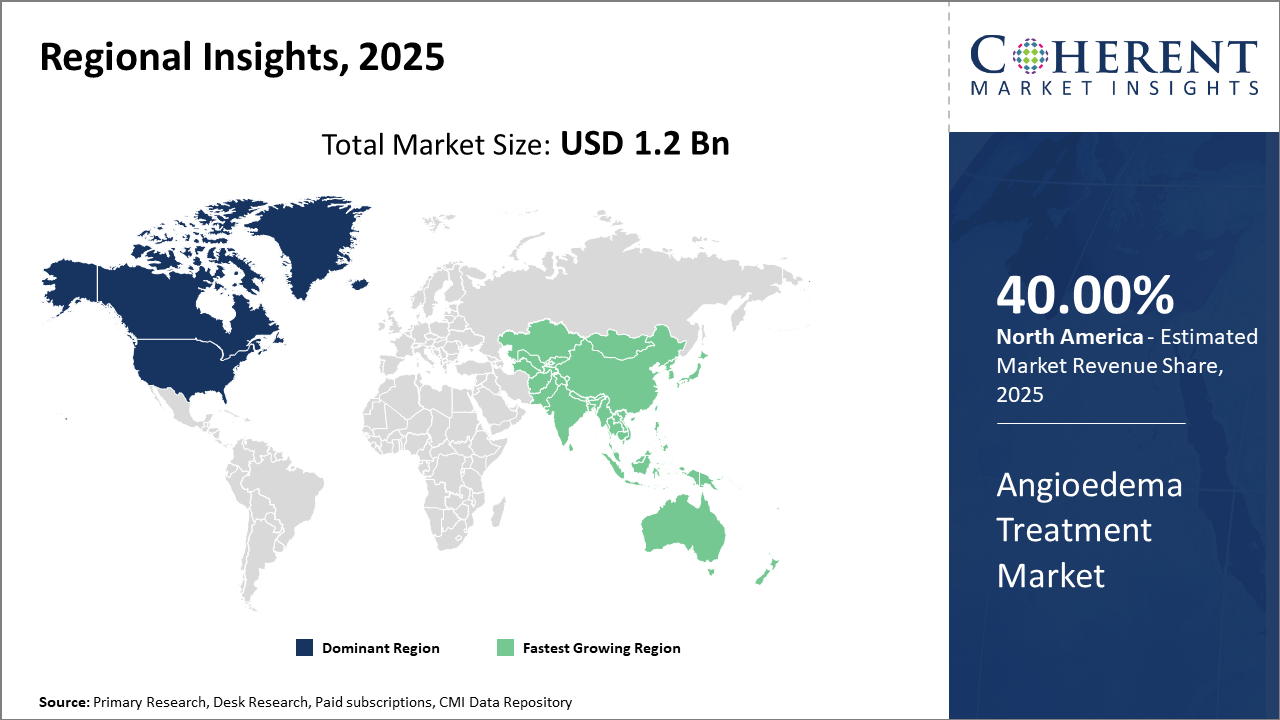
Regionally, North America leads the Angioedema Treatment Market with over 40% market share, underpinned by well-established healthcare infrastructure, advanced diagnostic facilities, and extensive R&D activities by market companies.
Asia Pacific emerges as the fastest-growing region, driven by improving healthcare access, increased disease awareness, and rising government initiatives supporting rare disease management.
Angioedema Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Angioedema Treatment Market Insights, By Treatment Type
The Plasma-Derived C1-inhibitors dominate the market share with approximately 45%. This dominance stems from their long clinical track record, broad reimbursement coverage, and proven efficacy in acute and prophylactic management, making them first-line options in many treatment protocols. The fastest-growing subsegment is Kallikrein Inhibitors, bolstered by recent approvals of oral formulations that offer greater patient convenience and potential for expanded indications beyond hereditary angioedema.
Angioedema Treatment Market Insights, By Disease Type
Hereditary Angioedema holds the leading market share, attributed to the higher diagnosis rates and established treatment paradigms. This segment benefits from increased genetic testing and standardized care pathways, accounting for a major portion of market revenue. Acquired Angioedema is the fastest-growing subsegment, especially in aging populations and patients with associated lymphoproliferative disorders, reflecting shifts in epidemiology and demand for tailored therapies.
Angioedema Treatment Market Insights, By Route of Administration
Intravenous administration commands the dominant share due to its historical use in delivering plasma-derived and recombinant therapies in hospital or clinical settings. However, the fastest-growing subsegment is Oral therapy, revolutionizing the market landscape through novel kallikrein inhibitors that offer the convenience of at-home treatment and improved adherence.
Angioedema Treatment Market Trends
Recent years have seen a paradigm shift in treatment modalities toward personalized medicine and non-invasive drug delivery in the market.
Oral kallikrein inhibitors and subcutaneous self-administration devices have improved patient adherence and widened treatment appeal. For example, BioCryst Pharmaceuticals’ oral treatment approval in 2024 marked a notable milestone, boosting adoption rates in North America and Europe.
The rise of biosimilars has introduced price competition, especially in Europe, driving more patient-centric pricing models.
Digitization in healthcare is another trend, featuring remote disease monitoring platforms integrated with angioedema therapeutics, facilitating real-time patient management and reducing hospital visits.
Telehealth models in developed markets have enhanced disease management efficiency and reduced treatment costs, as seen in U.S. healthcare providers’ adoption of these tools from 2023 onwards.
Angioedema Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Angioedema Treatment Market Analysis and Trends
In North America, the dominance in the Angioedema Treatment Market remains robust, accounting for over 40% market share. This region’s leadership is attributed to extensive R&D investments, advanced healthcare infrastructure, and strong payer support. The presence of significant market players headquartered here accelerates innovation and clinical trial activities. The U.S. alone contributes a major portion of the market revenue, supported by streamlined regulatory pathways and awareness campaigns enhancing disease diagnosis rates.
Asia Pacific Angioedema Treatment Market Analysis and Trends
The Asia Pacific region exhibits the fastest growth with a CAGR exceeding 11% influenced by rising healthcare expenditure, increasing patient awareness, and government health initiatives targeting rare diseases. Improving reimbursement frameworks in countries like China and India are propelling patient access to advanced biologics and novel therapies. Market companies have increasingly focused on expanding distribution networks and local partnerships to tap into the therapeutic demand surge.
Angioedema Treatment Market Outlook for Key Countries
USA Angioedema Treatment Market Analysis and Trends
The U.S. Angioedema Treatment Industry is the largest regionally, fueled by high prevalence of hereditary angioedema and increased health insurance coverage. Notable companies, including Takeda Pharmaceutical and CSL Behring, have introduced innovative therapies such as recombinant C1-inhibitors and oral kallikrein inhibitors, capturing significant market share. Between 2023 and 2025, the pace of clinical trials and drug approvals accelerated, expanding treatment options and enhancing patient outcomes. Additionally, government initiatives for rare disease research funding have played a pivotal role in sustaining industry share and business growth.
Germany Angioedema Treatment Market Analysis and Trends
Germany’s market is a key contributor in Europe, supported by a strong healthcare ecosystem and robust funding for rare diseases. The country benefits from favorable reimbursement policies, which have encouraged uptake of plasma-derived and recombinant therapies. Leading market companies maintain operations and clinical research hubs here to streamline distribution and regulatory compliance. Germany’s strategic collaborations with regional healthcare providers enhance patient access and have resulted in consistent market growth. Moreover, initiatives increasing awareness of angioedema subtypes have positively influenced diagnosis and treatment patterns in recent years.
Analyst Opinion
The increasing diagnosis rate of hereditary angioedema (HAE) is a critical demand-side driver reflected in the market size. For instance, in 2024, diagnostic rates in North America rose by over 15% due to improved genetic testing capabilities, driving market revenue. Additionally, the surge in emergency room visits for angioedema-related complications in Europe by 12% during 2023 signals heightened demand for acute-phase treatment options.
On the supply side, the expansion of biologics manufacturing capacity has contributed to market growth. A key example is the capacity ramp-up by several pharmaceutical entities in 2025, increasing global production output of C1-inhibitor therapies by nearly 20%. This flux positively impacted market share by ensuring drug availability in previously underserved regions in the Asia Pacific.
Pricing strategies influencing market dynamics have shown a crucial impact for both branded and biosimilar therapeutics. The entry of biosimilars in 2024 reduced average treatment costs by approximately 18% in developed countries, expanding accessibility and fostering business growth in the angioedema treatment industry.
Use case diversification with off-label adoption and new indications fuels market expansion. Clinical studies published in early 2025 demonstrated the effectiveness of specific kallikrein inhibitors in refractory cases, enhancing market scope and triggering competitive responses among market players to broaden product pipelines.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.4% | 2032 Value Projection: | USD 2.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Shire Pharmaceuticals, CSL Behring, Takeda Pharmaceutical Company, BioCryst Pharmaceuticals, KalVista Pharmaceuticals, Pharming Group N.V., Octapharma AG, HAE Pharma, Sobi, Novartis, Idorsia Pharmaceuticals, and Argenx NV. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Angioedema Treatment Market Growth Factors
The first key growth driver is the rising patient awareness coupled with improved diagnostic capabilities, which has led to greater identification of hereditary and acquired angioedema cases worldwide. Enhanced access to genetic testing and diagnostic tools in 2024 led to a 13% increase in diagnosed patients, fuelling prescriptions for specialty treatments. Secondly, technological innovations in biologics and small-molecule therapies are providing safer and more effective treatment options. For instance, the launch of an oral kallikrein inhibitor in 2025 expanded treatment options beyond traditional intravenous therapy, driving market growth by broadening patient accessibility. Thirdly, favorable reimbursement policies and increased healthcare spending in developed countries, particularly in the U.S. and EU, have enabled better patient access to high-cost therapies, supporting sustained market revenue growth.
Angioedema Treatment Market Development
In July 2025, the U.S. FDA approved Ekterly (sebetralstat), a novel plasma kallikrein inhibitor, as the first and only oral on-demand treatment for acute HAE attacks in patients aged 12 years and older. Ekterly showed in the KONFIDENT phase 3 trial and its extension (KONFIDENT-S) that it significantly eased attack symptoms, reduced severity, and resolved attacks more quickly compared to placebo.
In August 2025, the FDA approved Dawnzera, developed by Ionis Pharmaceuticals, as the first RNA-targeted prophylactic treatment for HAE in adults and adolescents (12+ years). In clinical studies (including OASISplus), it was shown to reduce the frequency of HAE attacks by up to ~94% depending on dosing interval.
Key Players
Major market players include:
Shire Pharmaceuticals
CSL Behring
Takeda Pharmaceutical Company
BioCryst Pharmaceuticals
KalVista Pharmaceuticals
Pharming Group N.V.
Octapharma AG
Sobi
Novartis
Idorsia Pharmaceuticals
Argenx NV
Competitive strategies have notably included portfolio diversification and strategic acquisitions; for example, Shire’s acquisition by Takeda in late 2024 enhanced its portfolio dominance in hereditary angioedema treatment, improving market share significantly in North America and Europe. BioCryst Pharmaceuticals’ recent licensing agreement for oral kallikrein inhibitors in 2025 increased its presence in emerging markets, resulting in a 25% revenue uptick in Asia Pacific in Q1 2025.
Angioedema Treatment Market Future Outlook
Future market growth will be propelled by ongoing R&D in monoclonal antibodies, small-molecule inhibitors, and gene therapies offering long-term control or potential cures. The focus will remain on personalized medicine, with treatment regimens tailored to individual genetic profiles and disease severity. Expansion into emerging markets and rising healthcare expenditure will increase accessibility to biologic therapies. Additionally, patient-centric models emphasizing self-administration, telehealth follow-ups, and digital monitoring tools will transform the management of chronic angioedema.
Angioedema Treatment Market Historical Analysis
The Angioedema Treatment Market was historically dominated by symptomatic therapies such as antihistamines and corticosteroids for allergic forms of the condition. The introduction of targeted biologics and C1 esterase inhibitor concentrates revolutionized treatment for hereditary angioedema (HAE), significantly improving patient outcomes. Regulatory approvals for subcutaneous and on-demand formulations enhanced convenience and adherence, while diagnostic advances allowed earlier and more accurate identification of HAE cases. Over time, pharmaceutical innovation and patient advocacy initiatives increased awareness and treatment access.
Sources
Primary Research interviews:
Immunologists
Allergists
Pharmaceutical R&D Experts
Clinical Trial Investigators
Databases:
FDA Orphan Drug Database
NCBI Clinical Data
Orphanet Rare Disease Database
Magazines:
PharmaTimes
BioPharma Dive
Drug Discovery & Development
Journals:
Journal of Allergy and Clinical Immunology
Clinical and Experimental Immunology
Allergy Journal
Therapeutics and Clinical Risk Management
Newspapers:
The Times of India (Health)
The Guardian (Medical Research)
STAT News (Biotech)
Associations:
American Academy of Allergy
Asthma & Immunology (AAAAI)
World Allergy Organization (WAO)
International HAE Association
European Academy of Allergy and Clinical Immunology (EAACI)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients