Angina Pectoris Treatment Market Size and Forecast – 2025 – 2032
The Global Angina Pectoris Treatment Market size is estimated to be valued at USD 9.45 billion in 2025 and is expected to reach USD 14.76 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.5% from 2025 to 2032.
Global Angina Pectoris Treatment Market Overview
Angina pectoris is treated to reduce chest pain and prevent heart attacks. Management includes lifestyle changes like quitting smoking, eating a heart-healthy diet, regular exercise, and stress reduction. Medications such as nitrates (e.g., nitroglycerin), beta-blockers, calcium channel blockers, antiplatelet agents (e.g., aspirin), and statins are commonly used to improve blood flow and reduce heart workload. In severe cases, procedures like angioplasty with stenting or coronary artery bypass grafting (CABG) may be needed. Controlling risk factors such as high blood pressure, diabetes, and high cholesterol is crucial. Regular follow-ups and adherence to treatment significantly improve outcomes and quality of life.
Key Takeaways
Pharmaceuticals dominate the treatment segment, driven principally by beta-blockers capturing approximately 58% of the market share, reflecting their enduring efficacy and clinician preference.
C- Combination therapies and interventional devices are emerging subsegments steering market growth strategies, as evidenced by robust clinical adoption rates in Europe and North America.
The Asia Pacific region is poised as a significant growth frontier, with India and China pushing market revenue expansions due to rising disease incidence and bolstered healthcare infrastructure.
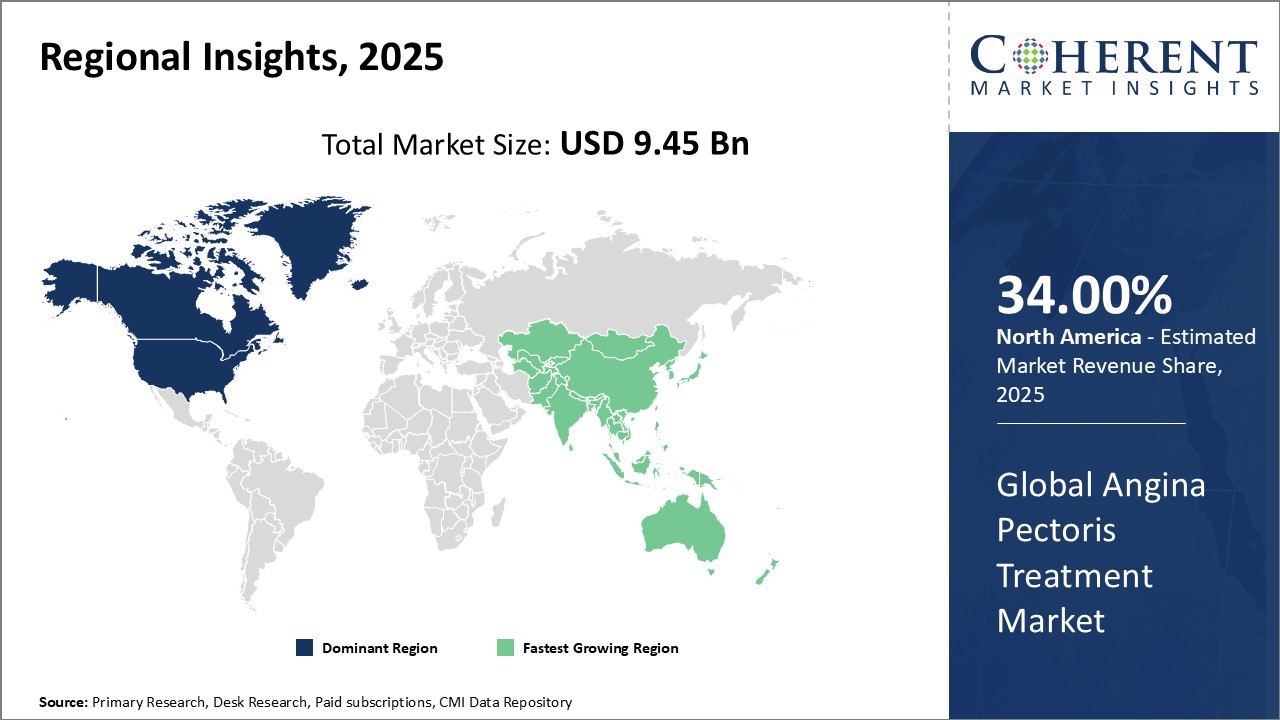
North America continues as the dominating region, contributing over 34% industry share, propelled by advanced healthcare systems and high adoption rates of novel therapeutics.
Angina Pectoris Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
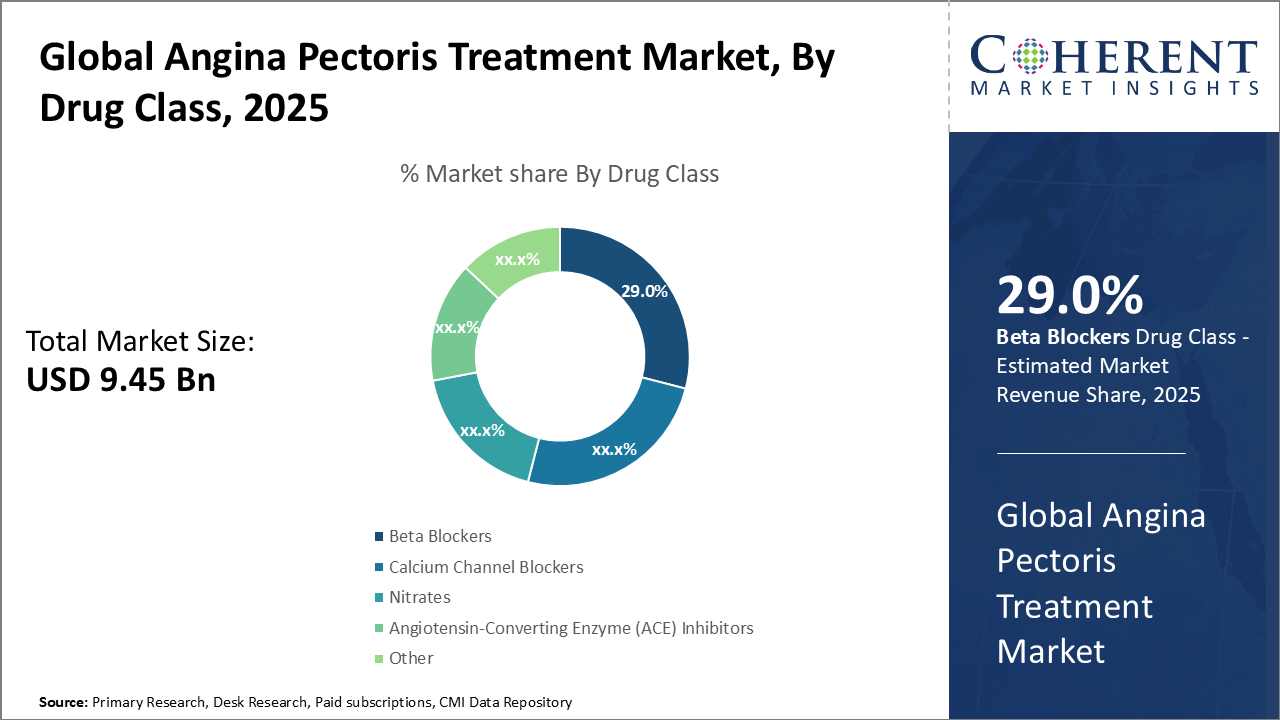
Angina Pectoris Treatment Market Insights, By Drug Class
Beta-Blockers hold the dominant share (29%) attributable to their extensive historical use and proven outcomes in angina symptom control. Calcium Channel Blockers are the fastest-growing class driven by effectiveness in reducing myocardial oxygen demand and favorable safety profiles, emerging as preferred options in patient subsets intolerant to beta-blockers. Nitrates, providing rapid symptomatic relief, maintain a steady share but are challenged by tolerance development, limiting long-term use.
Angina Pectoris Treatment Market Insights, By Treatment Type
In terms of treatment type, the Pharmaceuticals segment dominates the market share. This segment accounts for approximately 58% of total market revenue due to its widespread use as the first-line approach for angina management. Beta-blockers and nitrates remain staple treatments, but improved calcium channel blockers and novel agents are expanding treatment efficacy, resulting in their faster growth. Interventional Devices, notably advanced PCI tools, represent the fastest-growing subsegment as minimally invasive procedures gain traction for refractory cases, supported by data showing increased procedural volumes globally.
Angina Pectoris Treatment Market Insights, By End-User
Hospitals lead the market share, influenced by the high complexity of angina cases managed, availability of interventional devices, and inpatient treatment protocols. Hospitals also benefit from early diagnosis and integration with cardiac care units. Clinics cater mainly to chronic stable angina patients seeking routine pharmacological management and outpatient follow-ups, thus holding sizable revenue shares. Ambulatory Surgical Centers are witnessing growth due to the shift toward outpatient PCI interventions, reflected in increased procedural volumes. Home Care Settings represent an emergent segment fueled by digital health platforms facilitating remote patient monitoring and management, gaining momentum especially in developed regions.
Angina Pectoris Treatment Market Trends
Recent market trends in Angina Pectoris Treatment emphasize innovation in pharmaceutical formulations and device-based therapies complemented by digital health integration.
Enhanced efficacy and safety profiles of newer medications such as selective calcium channel blockers have improved patient adherence, validating shifts in prescribing behaviors documented in OECD health reports for 2025.
Technological advancements in PCI and EECP devices reflect a trend toward less invasive interventions, corroborated by European cardiology registries recording a 12% procedural increase.
Moreover, telemedicine's integration is reshaping market dynamics, enabling remote monitoring and intervention through platforms piloted in North America and parts of Europe, signifying enhanced patient outcomes and reduced costs.
Angina Pectoris Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Angina Pectoris Treatment Market Analysis and Trends
In North America, the dominance in the Angina Pectoris Treatment market is underpinned by robust healthcare infrastructure, widespread insurance coverage, and the presence of leading market companies. The U.S. accounts for the largest industry share at over 34%, driven by extensive adoption of novel therapeutic agents and procedural interventions. Government support for cardiovascular disease management and high R&D investments further consolidate this region’s market scope, with notable companies such as Pfizer and Johnson & Johnson actively driving product innovations and expansions.
Asia Pacific Angina Pectoris Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR exceeding 8%, fueled by increasing cardiovascular disease prevalence, rising healthcare expenditure, and expanding access in emerging nations like India and China. Government initiatives aimed at long term care and public health awareness campaigns have accelerated market penetration. Players such as AstraZeneca and Novartis have expanded footprints through partnerships and local manufacturing, fostering rapid business growth in the region.
Angina Pectoris Treatment Market Outlook for Key Countries
USA Angina Pectoris Treatment Market Analysis and Trends
The USA’s Angina Pectoris Treatment market is a primary revenue contributor, propelled by advanced healthcare policies, high healthcare expenditure exceeding USD 4 trillion annually, and significant emphasis on cardiovascular health. Clinical trials conducted within the U.S. in 2024 tested novel anti-anginal agents that demonstrated enhanced efficacy and safety, influencing regulatory approvals and commercial availability. Market companies have adopted aggressive marketing strategies and digital patient engagement initiatives that improved treatment adherence rates by 10%-12%, reinforcing the country’s leadership in market revenue and technological implementation.
India Angina Pectoris Treatment Market Analysis and Trends
India’s market is distinguished by rapid growth underpinned by increasing cardiovascular disease burden and expanding urban healthcare infrastructure. The government’s Pradhan Mantri Jan Arogya Yojana (PM-JAY) launched enhanced coverage options for chronic disease management in 2024, improving patient access to angina therapies. The market growth is further supported by increasing generic drug production capacity and rising awareness through targeted campaigns. Indian market players are investing in cost-effective treatment modalities, ensuring affordability and driving penetration into rural demographics, which collectively bolster the country’s strategic significance within the Asia Pacific market.
Analyst Opinion
A critical driver of market size is the rising incidence of chronic stable angina, particularly in aging populations. Notably, cardiovascular mortality data from the American Heart Association in 2024 highlighted a 3.2% annual increase in ischemic heart disease diagnoses among adults aged 65 and above, underscoring growing healthcare demand. This epidemiological trend places heightened pressure on pharmaceutical pipelines and intervention patterns, directly influencing market share.
Pricing and treatment accessibility remain pivotal supply-side factors. Recent adjustments in drug pricing models, such as the introduction of generic formulations of ranolazine in 2025, have expanded market revenue streams by providing cost-effective options, facilitating greater penetration in emerging markets. For instance, India reported a 15% growth in angina drug consumption following regulatory endorsement of generics, illustrating how pricing strategies shape market expansion.
Market growth also derives from technological advancements in device-based therapies including enhanced percutaneous coronary intervention (PCI) devices that promise better symptomatic control and reduced rehospitalization. A 2025 clinical registry from Europe noted a 12% increase in PCI adoption rates for refractory angina, augmenting interventional treatment revenue within the Angina Pectoris Treatment Market.
Demand-side indicators reveal diversified use cases across cardiology care settings, with increasing preference for combination therapy protocols. A survey conducted in 2024 among U.S. cardiologists showed 68% adoption of combined nitrates and beta-blocker regimens, underscoring evolving prescribing trends that accentuate the market’s share of pharmacological segments.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 9.45 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.5% | 2032 Value Projection: | USD 14.76 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | AstraZeneca, Novartis AG, Pfizer Inc., Bayer AG, Johnson & Johnson, Roche Holding AG, Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences Inc., Medtronic plc, Boston Scientific Corporation, and Terumo Corporation | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Angina Pectoris Treatment Market Growth Factors
The primary drivers include a rising global prevalence of cardiovascular diseases driven by aging populations and lifestyle disorders, which underscores sustained market growth. Second, advancements in pharmacological treatments such as optimized beta-blocker formulations and the introduction of novel calcium channel blockers have broadened therapeutic options, facilitating expanded treatment adoption.
Furthermore, increased healthcare spending in emerging economies—boosted by government initiatives promoting cardiovascular health—has widened patient access, exemplified by Brazil’s national health policy enhancements in 2024 that increased angina treatment reimbursements by 18%. Finally, growing demand for minimally invasive interventional therapies has bolstered market revenue, as evidence from the U.K. indicates a 10% annual rise in PCI procedures for angina management between 2023 and 2025.
Angina Pectoris Treatment Market Development
In April 2025, the Mayo Clinic published a study in JACC: Cardiovascular Interventions highlighting the potential of an hourglass-shaped coronary microvascular stent. This innovative device aims to improve blood flow and alleviate angina symptoms in patients with coronary microvascular disease, offering a new treatment avenue for this challenging condition.
In March 2025, the American College of Cardiology (ACC) and American Heart Association (AHA) released updated guidelines for the management of acute coronary syndromes, which include recommendations for the treatment of stable angina. These guidelines emphasize the importance of a patient-centered approach, integrating pharmacological, interventional, and lifestyle strategies to reduce cardiovascular risk and improve patient outcomes.
Key Players
Major market players include AstraZeneca, Novartis AG, Pfizer Inc., Bayer AG, Johnson & Johnson, Roche Holding AG, Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences Inc., Medtronic plc, Boston Scientific Corporation, and Terumo Corporation.
Competitive strategies prominently revolve around mergers and acquisitions, strategic partnerships for pipeline enhancement, and geographic expansion. For example, AstraZeneca’s 2025 alliance with a key Japanese pharmaceutical firm led to a 7.8% increase in Asia Pacific market penetration, highlighting effective regional growth strategies. Additionally, Medtronic’s launch of next-generation PCI devices in 2024 boosted its market share within interventional therapy segments by 5%, demonstrating innovation-driven revenue impact.
Angina Pectoris Treatment Market Future Outlook
The treatment landscape for angina pectoris is expected to continue evolving, supported by innovations in personalized medicine and novel drug delivery systems. Increasing awareness of cardiovascular health, combined with lifestyle-related risk factors, will drive the need for more effective therapies. Emerging economies, particularly in Asia and Europe, are likely to see significant growth in access to advanced treatments, aided by improvements in healthcare infrastructure. Overall, the demand for effective angina management solutions is projected to remain strong, offering opportunities for new therapies and improved treatment approaches worldwide.
Angina Pectoris Treatment Market Historical Analysis
The understanding and treatment of angina pectoris have evolved significantly over time. The term "angina pectoris" was first introduced by the English physician William Heberden, who described the condition as a sensation of tightness or pressure in the chest. In the following years, Thomas Lauder Brunton identified the vasodilatory effects of amyl nitrite, which led to its use in relieving angina symptoms. Later, William Murrell demonstrated the effectiveness of nitroglycerin in treating angina, marking a major advancement in pharmacological therapy.
Over the subsequent decades, further innovations transformed angina management. The introduction of beta-blockers provided an important tool to reduce myocardial oxygen demand and control symptoms. Non-invasive therapies, such as Enhanced External Counterpulsation (EECP), emerged as alternatives for patients who were not suitable for surgical interventions. Advances in diagnostic techniques, including coronary angiography and non-invasive imaging, have greatly improved the understanding and management of angina, allowing for more targeted and effective treatment strategies.
Primary Research interviews:
Cardiologists
Cardiac Surgeons
Clinical Pharmacologists
Hospital Administrators
Databases:
National Center for Biotechnology Information (NCBI)
World Health Organization (WHO) Database
Centers for Disease Control and Prevention (CDC) Database
Magazines:
Cardiology Today
Heart Health Magazine
American Heart Journal Insights
Prevention (Heart & Lifestyle Section)
Health & Nutrition
Journals:
Journal of the American College of Cardiology (JACC)
European Heart Journal
Circulation
Cardiovascular Drugs and Therapy
International Journal of Cardiology
Newspapers:
The New York Times (Health Section)
The Washington Post (Medical & Health)
Hindustan Times (Health & Lifestyle)
News18 (Health Updates)
Associations:
American Heart Association (AHA)
International Society for Heart Research (ISHR)
World Heart Federation (WHF)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients