Androgenetic alopecia (AGA) is a patterned hair loss occurring due to systemic androgen and genetic factors. It is the most common cause of hair loss in both genders. It is characterized by follicular miniaturization in a patterned hair loss occurring due to systemic androgen and genetic factors. The increasing incidence of androgenetic alopecia is expected to boost the market growth. According to the National Center for Biotechnology Information (NCBI), in August 2015, global prevalence of severe androgenetic alopecia (AGA) was 15.33% overall and varied significantly by geographical region. The risk of having severe androgenetic alopecia (AGA) increased by 1.092 for per year for the 30 and 40 age group.
The global androgenetic alopecia treatment market is estimated to be valued at US$ 1,607.47 million in 2020 and is expected to exhibit a CAGR of 5.2% during the forecast period (2020-2027).
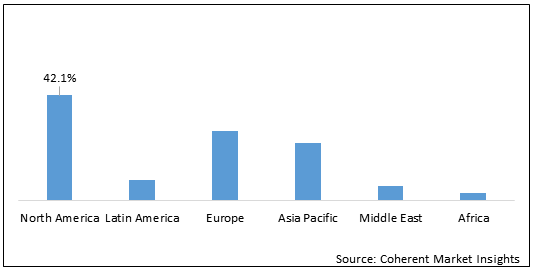
Figure 1. Global Androgenetic Alopecia Treatment Market Share (%), by Region, 2020
To learn more about this report, Download Free Sample
Product development and ongoing clinical trials are expected to propel growth of the global androgenetic alopecia treatment market.
Development of drugs for androgenetic alopecia treatment is expecting to boost the market growth over the forecast period. For instance, Cairo University is conducting clinical trial of topical Cetirizine for the treatment of androgenetic alopecia in females. The trial is in Phase II/III evaluating the efficacy and tolerability of topical cetirizine in female patients with androgenetic alopecia (AGA). This interventional trial started in February 2020 and the study expected completed in December 2021.
However, side effects of androgenetic alopecia treatment are expected to hinder the market growth. According to the US Pharmacist, a clinically-focused pharmacy publication, published an article- Treatment Options for Androgenetic Alopecia, in 2018 that medication used in for the treatment of androgenetic alopecia has many side effects. For example, topical Minoxidil can cause local erythema and pruritus and Finasteride can cause dizziness, orthostatic hypotension and others.
Androgenetic Alopecia Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2020: | US$ 1,607.47 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 5.3% | 2027 Value Projection: | US$ 2,298.34 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Histogen Inc., Cipla Limited, Aclaris Therapeutics, Inc., Merck & Co., Inc., Daiichi-Sankyo Co., Ltd., Johnson and Johnson Services, Inc., Lexington International LLC, Vita-Cos-Med Klett-Loch GmbH, PureTech, Vitabiotics, Dr. Reddy’s Laboratories, HCell Inc., Follica, Inc. and Ranbaxy Laboratories Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
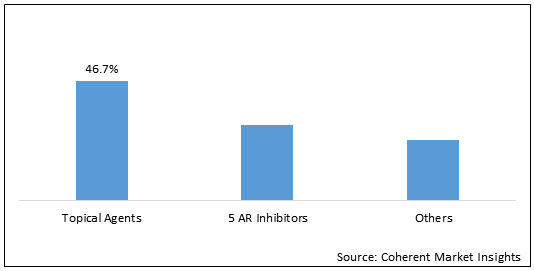
Figure 2. Global Androgenetic Alopecia Treatment Market Share (%), by Drug Type, 2020
To learn more about this report, Download Free Sample
Research and development of treatment for androgenetic alopecia in North America is expected to boost the market growth.
North America is expected to hold dominant position in the global androgenetic alopecia treatment market owing to research and development of treatments for androgenetic alopecia in the region. For instance, on June 4, 2020, Follica, Inc., a biotechnology company developing a regenerative platform designed to treat androgenetic alopecia, and other related conditions, announced positive feedback from the U.S. Food and Drug Administration (FDA) for its FOL-004 Phase II released in December 2019. The company advanced its lead program, FOL-004 into Phase III development, following a successful safety and efficacy optimization study for the treatment of hair loss in male androgenetic alopecia.
Global Androgenetic Alopecia Treatment Market – Impact of Coronavirus (COVID-19) Pandemic
Nationwide lockdowns resulting from COVID-19 in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare is one such sector which has been impacted significantly by the COVID-19 pandemic.
The lockdown in various countries due to the pandemic has placed an economic burden on the healthcare sector. Moreover, the coronavirus pandemic has hampered the development, production, and supply of drugs and other healthcare products and affected growth of healthcare businesses of various companies across the globe.
As a result, the impact of the COVID-19 pandemic is also expected to limit growth of the global androgenetic alopecia treatment drugs market during the forecast period.
Key Players
Major players operating in the global androgenetic alopecia treatment market are Histogen Inc., Cipla Limited, Aclaris Therapeutics, Inc., Merck & Co., Inc., Daiichi-Sankyo Co., Ltd., Johnson and Johnson Services, Inc., Lexington International LLC, Vita-Cos-Med Klett-Loch GmbH, PureTech, Vitabiotics, Dr. Reddy’s Laboratories, HCell Inc., Follica, Inc. and Ranbaxy Laboratories Ltd. among others.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients