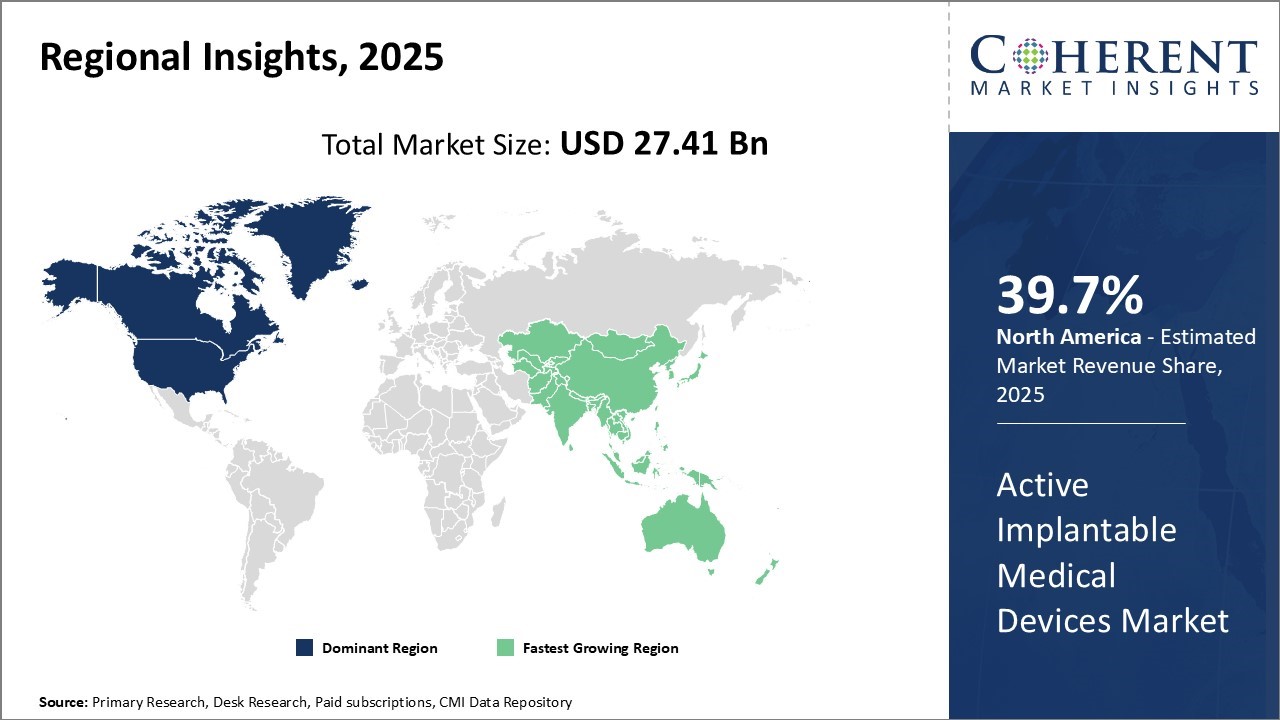
Global active implantable medical devices market is estimated to be valued at USD 27.41 Bn in 2025 and is expected to reach USD 45.21 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.4% from 2025 to 2032.
To learn more about this report, Download Free Sample
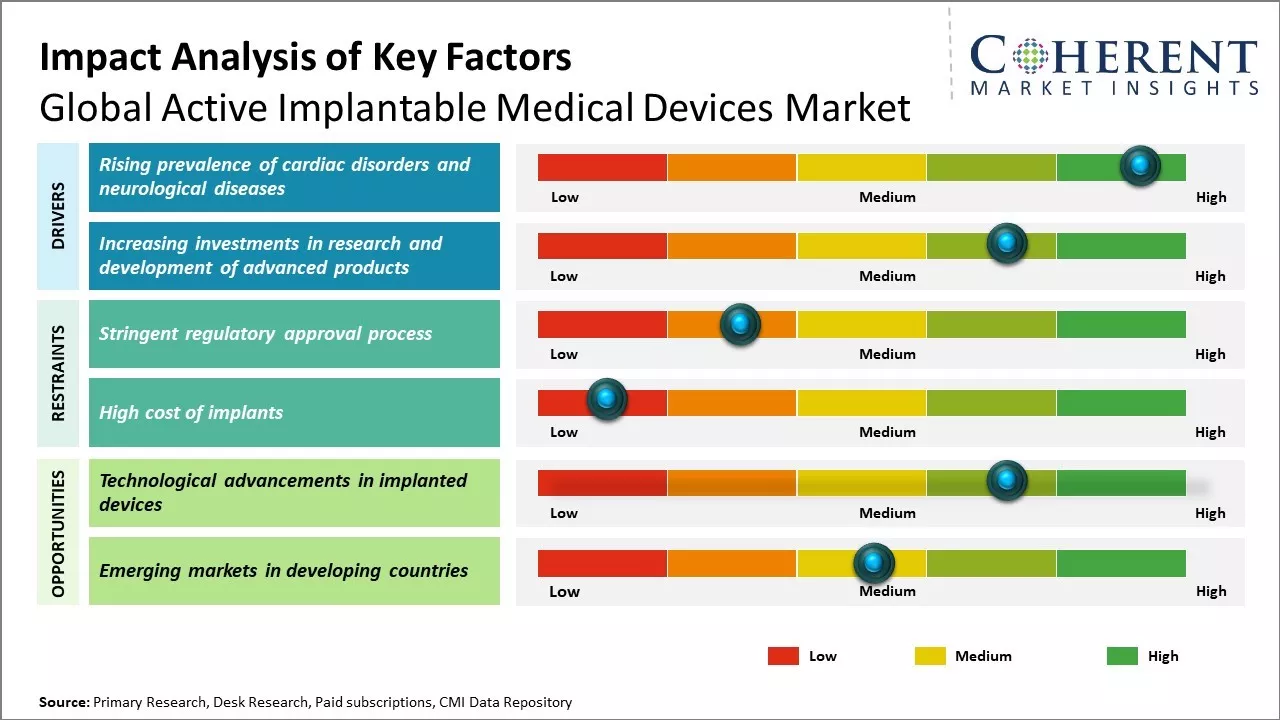
Global active implantable medical devices market growth is driven by the increasing prevalence of cardiac and neurological disorders. Rising aging population, growing awareness about advanced treatment procedures and favorable reimbursement policies can also drive the market growth. Technological advancements in these medical devices with focus on miniaturization and improvements in battery life have increased preference for active implantable devices over other treatment options. High cost of these devices and procedural complications can hamper the market growth.
|
Current Events |
Description and its impact |
|
Geopolitical and Regulatory Developments |
|
|
Technological Innovations and R&D Advances |
|
|
Competitive Landscape and Market Consolidation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Implantable Cardioverter Defibrillators segment is estimated to contribute the highest market share of 35.5% in 2025. Rising incidence of cardiovascular diseases such as sudden cardiac arrest, heart failure, and abnormal heart rhythms has significantly increased the risk of life-threatening cardiac events among the general population. ICDs have proven highly effective at detecting and responding to dangerous arrhythmias, thus, making these the treatment of choice for many patients at high risk. Ongoing technological innovations have enhanced the capabilities of modern ICDs.
Newer devices are smarter, smaller, and more reliable than earlier models. Features like remote monitoring allow devices to continually transmit battery status and arrhythmia data to clinicians without requiring frequent office visits. This improves patient care while reducing healthcare costs. ICDs also benefit from having a longer lifespan than alternative treatments such as medications. For instance, in October 2023, Medtronic plc, a global leader in healthcare technology, secured U.S. Food and Drug Administration (FDA) approval for its Aurora EV-ICD™ MRI SureScan™ (Extravascular Implantable Cardioverter-Defibrillator) and Epsila EV™ MRI SureScan™ defibrillation led to treat dangerously fast heart rhythms that can cause sudden cardiac arrest (SCA). This is further propelling the active implantable medical devices market share.
Hospitals segment is estimated to contribute the highest market share of 51.7% in 2025. Hospital facilities are best equipped with resources needed for complicated implantation processes. Cardiothoracic operating theatres provide ideal sterile environments while equipment like fluoroscopy machines enables real-time visibility. Dedicated electrophysiology labs streamline device testing. Post-op monitoring capabilities minimize risks. Hospitals concentrate cardiac specialists with expertise in device therapies. Electrophysiologists, cardiac surgeons and other clinicians extensively trained in procedures like complex ICD implants are generally based in hospitals. Their collective experience handling diverse cases improves outcomes. Hospitals offer infrastructure for potential complications. Given the invasive nature of surgeries, rare issues may occasionally arise requiring advanced intervention beyond outpatient clinics' scope. For instance, in February 2025, CARMAT, the creator and developer of the world’s most advanced total artificial heart, announced that it has completed 100 implants at the university hospitals of Lille and Dijon. The device offers a therapeutic alternative for patients with advanced biventricular heart failure.
To learn more about this report, Download Free Sample
The North American Active Implantable Medical Devices (AIMD) industry is rapidly expanding, driven by several key factors. Excellent healthcare infrastructure and a high prevalence of chronic diseases, especially cardiovascular and neurological conditions, fuel this growth. Technological advancements, including artificial intelligence (AI) and telemedicine integration, enhance device performance and patient care. Additionally, favorable reimbursement policies and a strong regulatory framework support active implantable medical devices market revenue. Although high device costs and regulatory challenges limit accessibility, ongoing research and development continuously drive innovation in the AIMD sector.
For instance, in March 2023, Resonant Link, a U.S.-based company, introduced a 15-minute wireless charger for implantable medical devices, including titanium-can implants. This technology allows patients to recharge their titanium-can-based implants within minutes without interrupting their daily activities.
Asia Pacific is rapidly improving its healthcare infrastructure, with new hospitals and specialized cardiac clinics, increasing the use of active implantable medical devices. Growing investments in medical technology and facilities expand access to advanced treatments, especially in emerging economies like India and China, thereby enhancing patient care quality. The region is experiencing a rise in chronic diseases, including cardiovascular and neurological disorders. This surge in demand for devices such as pacemakers and implantable cardioverter defibrillators drives the active implantable medical devices market growth as more patients pursue long-term therapies to manage their health conditions. For instance, in February 2025, Olympus Corporation of Asia Pacific (Olympus APAC) unveiled its iTind device, an implantable device in South Korea to treat enlarged prostates resulting from benign prostatic hyperplasia.
AI and telehealth enhance AIMDs by enabling real-time monitoring, predictive diagnosis, and personalized therapy. For example, Boston Scientific develops AI-enabled deep brain stimulation systems that deliver tailored treatment for Parkinson’s patients, improving outcomes. The U.S. market for neurostimulation devices, including spinal cord and deep brain stimulators, grows rapidly as chronic pain and neurological disorders become more prevalent.
In January 2025, Sequana Medical has announced that the US FDA has approved the alfapump—the first active implantable medical device in the country that automatically and continuously drains ascites from the abdomen into the bladder. Such developments are supporting the active implantable medical devices market demand.
The Indian government is building a ₹440 crore medical device park in Noida, covering 350 acres. This initiative boosts domestic manufacturing of AIMDs like pacemakers, implants, and diagnostic equipment, reducing import reliance and strengthening local production capacity. India’s aging population increases chronic conditions such as cardiovascular disease and musculoskeletal disorders, driving higher demand for AIMDs like pacemakers, hip replacements, and breast implants, which further propels the active implantable medical devices market share.
To learn more about this report, Download Free Sample
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 27.41 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.4% | 2032 Value Projection: | USD 45.21 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
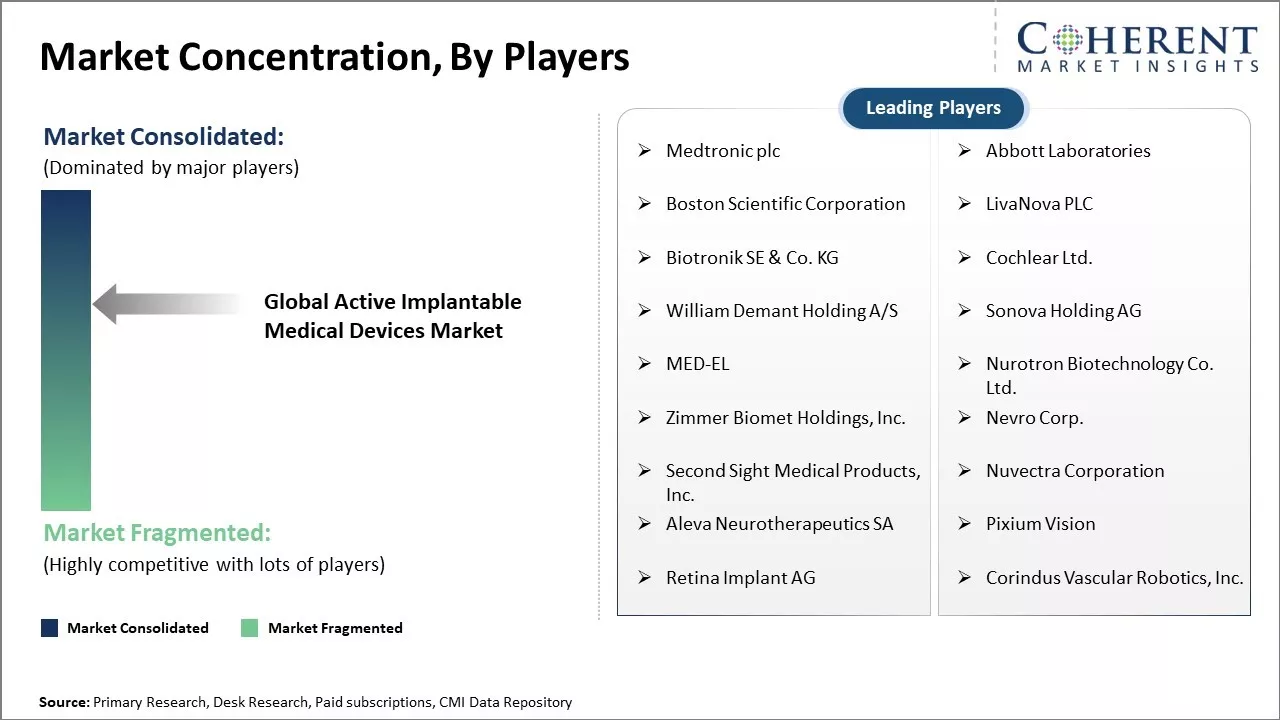
Medtronic plc, Abbott Laboratories, Boston Scientific Corporation, LivaNova PLC, Biotronik SE & Co. KG, Cochlear Ltd., William Demant Holding A/S, Sonova Holding AG, MED-EL, Nurotron Biotechnology Co. Ltd., Zimmer Biomet Holdings, Inc., Nevro Corp., Second Sight Medical Products, Inc., Nuvectra Corporation, Aleva Neurotherapeutics SA, Pixium Vision, Retina Implant AG, Corindus Vascular Robotics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The AIMD market is seeing increased integration of AI and digital health technologies. Devices are now capable of real-time data analysis, predictive diagnostics, and personalized treatment adjustments. AI enhances functionality in devices like pacemakers and neurostimulators, while app-based monitoring enables remote patient care. These innovations improve clinical decision-making, reduce hospital visits, and support chronic disease management. The shift toward intelligent, connected systems is reshaping how patients and healthcare providers interact with implantable medical technologies.
In February 2025, Biotronik announced it will divest its Vascular Intervention (VI) business to Teleflex as part of a strategic shift to focus on active implantable devices and digital healthcare. The company aims to advance in areas such as AI, remote patient monitoring, and connected health platforms.
Healthcare providers are favoring minimally invasive implantation procedures for AIMDs, reducing patient recovery time and lowering surgical risks. This trend is driving demand for smaller, more compact devices that can be implanted through less invasive techniques. Innovations in catheter-based delivery systems and robotic-assisted surgery are supporting this shift. As hospitals and patients prioritize reduced trauma and faster outcomes, manufacturers are responding with sleek, efficient designs that align with the needs of modern surgical practices.
The Active Implantable Medical Devices (AIMD) market is entering a critical transformation phase—
Healthcare providers are no longer satisfied with devices that simply maintain cardiac rhythm or modulate nerve signals. They demand proactive systems—ones that detect deterioration before it becomes an emergency. Consider Boston Scientific’s Vercise Genus deep brain stimulation system, which utilizes directional leads and integrated Bluetooth to fine-tune Parkinson’s treatment in real time. It is one of the few AIMDs responding to patient needs on a dynamic, daily basis. Yet most implants still function in a reactionary, static mode. That is no longer acceptable.
Another challenge in this market is device longevity and patient burden. The current standard battery life for pacemakers is 5–10 years. But each replacement poses infection risks, anesthesia exposure, and cost implications. Abbott’s Aveir VR Leadless Pacemaker, approved recently in the U.S., is a clear example of what the future looks like—miniaturized, leadless, retrievable, and designed for longevity. With fewer complications and no visible scarring, it is meeting both clinical and psychological needs that have long gone ignored.
However, technology alone doesn’t solve inequality. Emerging economies like India and Brazil still face a harsh economic divide where AIMDs—often priced over $20,000—are out of reach for the majority. Government-led initiatives like India’s Noida Medical Device Park are a promising step, but cost-effective design innovation remains absent. Until someone builds a sub-$5,000 pacemaker that doesn’t compromise on safety or intelligence, we’re just innovating for the top 10% of the population.
Moreover, regulatory dynamics are changing in favor of disruption. The FDA’s Breakthrough Device Designation pathway, which fast-tracked the approval of devices like LivaNova’s SenTiva DUO for drug-resistant epilepsy, shows that regulators are open to speed—if the science is sound. The opportunity here is clear: companies with data-backed innovation can now move faster than ever, but only if they can also prove real-world utility, not just technical novelty.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients