Absorbable Nasal Implant Devices Market Size and Forecast – 2026 – 2033
The Global Absorbable Nasal Implant Devices Market size is estimated to be valued at USD 320 million in 2026 and is expected to reach USD 590 million by 2033, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2026 to 2033.
Global Absorbable Nasal Implant Devices Market Overview
Absorbable nasal implant devices are minimally invasive medical products designed to support nasal airway structures. These implants are made from bioabsorbable polymers that provide temporary mechanical support to nasal cartilage and gradually dissolve within the body. They are used primarily to treat nasal valve collapse and improve airflow. The devices are inserted using specialized delivery systems during outpatient procedures. Key product benefits include reduced invasiveness, structural support, and natural absorption over time.
Key Takeaways
Nasal valve collapse remains the leading application segment, reflecting expanding treatment adoption across major markets. Hospitals continue to be the primary end users due to established surgical capabilities and reimbursement systems supporting complex procedures.
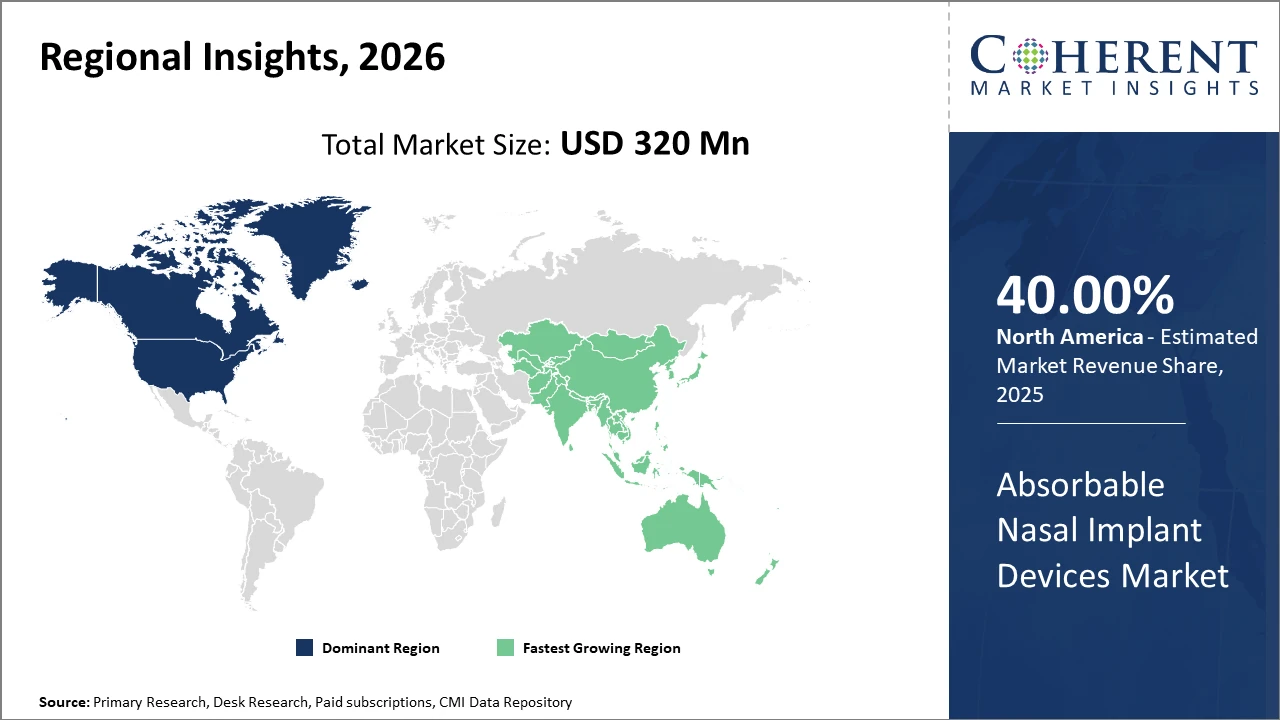
Regionally, North America leads the market share with over 40%, driven by advanced healthcare infrastructure and policy support.
Meanwhile, Asia Pacific registers the fastest market growth rate, owing to rising patient awareness and expanding ENT surgical facilities in emerging economies like India and China.
Europe maintains steady growth bolstered by ongoing innovation and reimbursement reforms.
Absorbable Nasal Implant Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
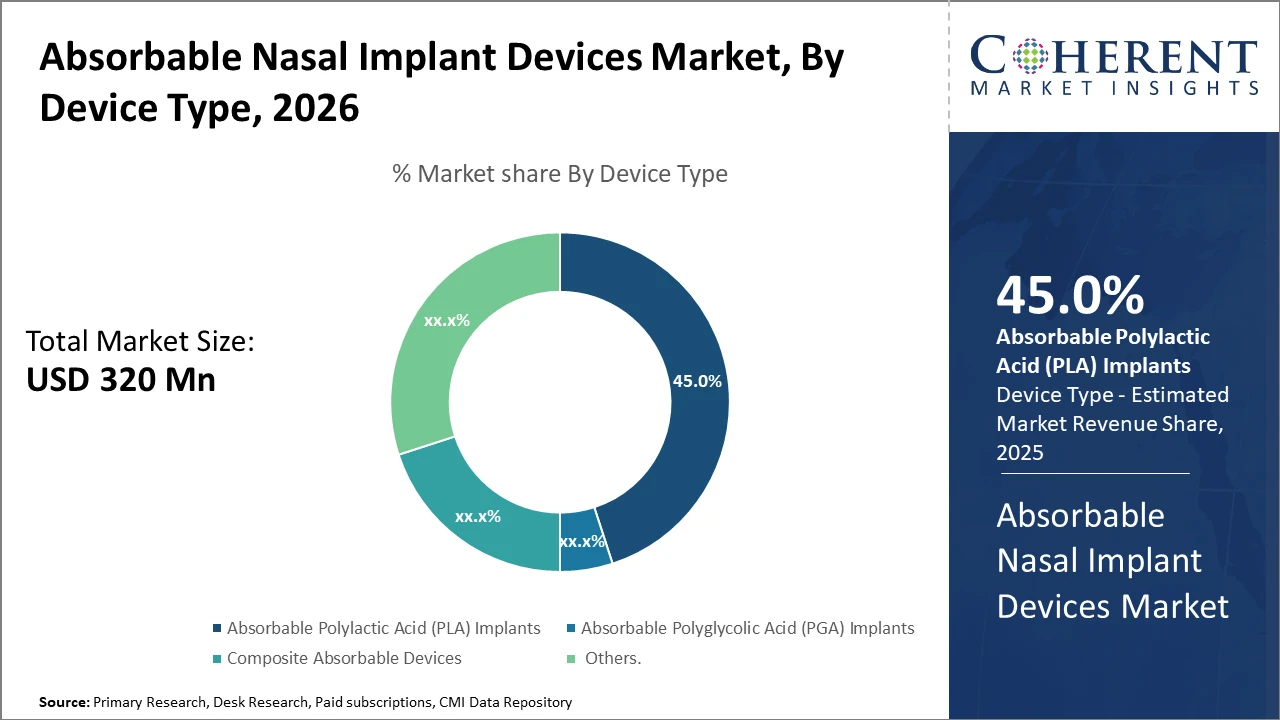
Absorbable Nasal Implant Devices Market Insights, By Device Type
Absorbable PLA Implants dominate the market share. PLA implants are preferred because of their favorable degradation rate, biocompatibility, and established clinical efficacy, making them the gold standard in nasal airway reconstruction. Their uniform resorption profile ensures predictable patient outcomes, which is crucial in minimizing revision surgery rates. Composite Absorbable Devices represent the fastest-growing subsegment, as they combine PLA or PGA matrices with bioactive ceramics or antimicrobial agents, promoting enhanced tissue integration and lowering infection risks. The increasing trend of customizing composite implants to patient-specific anatomical requirements is driving this subsegment’s rapid expansion. PGA Implants, while effective in certain applications, show faster degradation rates, limiting their use in long-term nasal structural support.
Absorbable Nasal Implant Devices Market Insights, By Application
Nasal Valve Collapse commands the dominant market share, given its high prevalence and the proven efficacy of absorbable implants in providing long-term structural support during minimally invasive procedures. The increasing incidence of nasal valve dysfunction in aging populations and post-trauma patients fuels continued demand. The fastest-growing application is Chronic Rhinosinusitis, driven by rising diagnosis rates and the need for implant solutions that facilitate sinus drainage and symptomatic relief without extensive surgery. Deviated Septum Correction remains steady, with moderate growth owing to surgical preferences often favoring traditional techniques, although absorbable devices are gaining traction in revision surgeries.
Absorbable Nasal Implant Devices Market Insights, By End-User
Hospitals dominate the market share. The comprehensive infrastructure and established reimbursement mechanisms in hospitals make them the preferred setting for complex nasal surgeries requiring absorbable implants, ensuring better procedural outcomes and patient follow-up care. Ambulatory Surgery Centers form the fastest-growing subsegment due to the growing trend of outpatient procedures, cost-effectiveness, and patient convenience. ASCs are increasingly equipped to perform functional nasal surgeries with absorbable implants, driven by technological advancements and streamlined surgical workflows. Specialized ENT Clinics provide targeted services but have a smaller market share due to limitations in handling severe cases.
Absorbable Nasal Implant Devices Market Trends
Recent market trends in absorbable nasal implant devices include substantial integration with advanced biodegradable materials, such as composite polymers that promote better tissue integration.
For example, clinical studies in 2025 highlight outcomes where composite implants reduced healing times by 18% compared to standard PLA devices.
Moreover, digital surgical planning software adoption is evolving rapidly—with a global increase of nearly 22% in procedural usage—facilitating more precise implant placements.
Adoption of outpatient nasal surgeries is on the rise with ambulatory surgery centers emerging as key hubs due to cost-effectiveness and patient convenience, particularly in North America.
Absorbable Nasal Implant Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Absorbable Nasal Implant Devices Market Analysis and Trends
In North America, the absorbable nasal implant devices market commands over 40% share, bolstered by highly developed healthcare systems, strong reimbursement policies, and significant R&D investments in ENT surgical innovations. The region’s well-established hospital infrastructure and the presence of major market players also contribute to sustained leadership. For instance, the U.S. saw a 15% increase in functional nasal procedures in 2024, supporting robust market revenue growth.
Asia Pacific Absorbable Nasal Implant Devices Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with a CAGR exceeding 10%, driven by expanding ENT surgical facilities, rising patient awareness, and favorable government healthcare initiatives in India and China. The region’s cost-effective manufacturing and increasing collaborations with global medical device companies fuel this rapid expansion. In 2025, India’s government healthcare spending on ENT procedural infrastructure increased by 14%, accelerating market penetration.
Absorbable Nasal Implant Devices Market Outlook for Key Countries
USA Absorbable Nasal Implant Devices Market Analysis and Trends
The USA’s market dominates due to advanced healthcare infrastructure and significant procedural volumes in nasal reconstructive surgeries. Major players like MedTech Biosurgical Inc. and Rhinoplasty Innovations Ltd. have concentrated R&D operations in the U.S., launching next-gen absorbable implants aligning with value-based healthcare. Government initiatives to expand outpatient surgical centers have facilitated market accessibility and revenue growth. More than 160,000 nasal surgeries in 2025 alone underscore the strong demand, complemented by reimbursement policies incentivizing absorbable device usage.
India Absorbable Nasal Implant Devices Market Analysis and Trends
India's market is rapidly emerging, catalyzed by increasing ENT patient awareness, expanding hospital infrastructure, and government healthcare spending focused on surgical advancements. Local companies are collaborating with international technology providers to enhance product offerings, including biodegradable nasal implants tailored to regional anatomical needs. The rise in private hospital capacity and outpatient surgical services contributes to the swift adoption of absorbable nasal implants. The market’s growth is further encouraged by cost-effective device manufacturing and improving healthcare insurance coverage, making India a pivotal growth frontier in this domain.
Analyst Opinion
The market revenue is primarily influenced by the rising number of nasal airway reconstruction procedures globally. For instance, in 2024, over 150,000 functional nasal surgeries were reported in North America alone, contributing to significant device demand. Furthermore, 2025 projections for Asia Pacific estimate surgical volumes to increase by 12%, reflecting increased market share in this region.
Material Innovations Accelerating Product Adoption: Advancements in biodegradable polymers such as polylactic acid and polyglycolic acid have led to improvements in device performance and patient outcomes. Clinical trials conducted in 2025 demonstrated a 25% reduction in post-operative complications when absorbable implants were used compared to non-absorbable options, underlining a positive shift in market dynamics.
Pricing Trends and Reimbursement Policies Influencing Market Growth: Market analysis indicates that favorable reimbursement frameworks in countries like Germany and the U.S. have positively impacted device pricing strategies, facilitating broader physician adoption. In 2024, pricing adjustments aligned with value-based healthcare models contributed to a 7% increase in procedure affordability in major markets.
Expansion in Outpatient Settings Enhances Market Scope: The shift from inpatient to outpatient nasal surgeries has expanded market opportunities. The rise of ambulatory surgery centers in 2025, particularly in the U.S. and Canada, accounted for a 15% increase in procedural throughput, thereby stimulating demand for absorbable nasal implant devices.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 320 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.3% | 2033 Value Projection: | USD 590 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | BioSculpt Implants, RespiraMed Systems, MedPoly Implants Pvt. Ltd., CAIRE Implants, OrthoNasal Medical, SinusTech Associates, Pioneer Nasal Devices, OptiNose Therapeutics, Advanced ENT Solutions, MedVance Implants. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Absorbable Nasal Implant Devices Market Growth Factors
The widespread increase in chronic nasal disorders such as nasal valve collapse and chronic rhinosinusitis propels demand for absorbable nasal implant devices, fostering sustainable market growth. Second, technological advancements in biodegradable materials continue to enhance device safety and efficacy, as evidenced by improved clinical outcomes reported in 2025, accelerating market adoption. Third, increasing patient preference for minimally invasive procedures coupled with enhanced reimbursement policies in developed economies drives the scope and accessibility of these implants. Lastly, expansion of outpatient surgical facilities is increasing procedural volumes, offering wider market penetration. For example, ambulatory centers in the U.S. have recorded a 14% surge in nasal implant device procedures in recent years.
Absorbable Nasal Implant Devices Market Development
In April 2024, Integra LifeSciences completed its USD 1 billion acquisition of Acclarent from Johnson & Johnson, significantly strengthening its position in the ENT market. The acquisition expanded Integra’s portfolio of minimally invasive sinus dilation and nasal implant technologies, enabling broader procedural offerings for chronic sinusitis and related ENT conditions while accelerating growth in its tissue technologies and specialty surgery segments.
In 2022, Medtronic completed its acquisition of Intersect ENT, bringing the PROPEL and SINUVA drug-eluting sinus implant product lines into its ENT portfolio. This move enhanced Medtronic’s capabilities in treating chronic sinus disease by combining implantable, steroid-eluting technologies with its existing surgical and navigation solutions, supporting improved postoperative outcomes and long-term disease management.
Key Players
Leading Companies of the Market
BioSculpt Implants
RespiraMed Systems
MedPoly Implants Pvt. Ltd.
CAIRE Implants
OrthoNasal Medical
SinusTech Associates
Pioneer Nasal Devices
OptiNose Therapeutics
Advanced ENT Solutions
MedVance Implants
Competitive dynamics reveal that MedTech Biosurgical Inc. recently expanded its product portfolio by acquiring innovative absorbable implant technologies, boosting its market share by 5% in 2024. BioNasal Systems implemented a targeted regional market growth strategy focusing on Asia Pacific, resulting in a 20% revenue increase in 2025 through new distributor partnerships.
Absorbable Nasal Implant Devices Market Future Outlook
Future growth in the absorbable nasal implant devices market will be driven by patient preference for minimally invasive procedures. Technological advancements will improve implant design, durability, and ease of placement. Expansion into outpatient settings will increase accessibility. Rising awareness of nasal valve collapse and sleep-related breathing disorders will support demand. The market will continue to benefit from innovations that improve patient comfort and clinical outcomes.
Absorbable Nasal Implant Devices Market Historical Analysis
The absorbable nasal implant devices market emerged as an alternative to traditional surgical interventions for nasal airway obstruction. Historically, treatment relied on invasive surgeries with longer recovery times. The introduction of bioabsorbable implants provided a minimally invasive solution that offered temporary structural support. Early adoption focused on specialized ENT practices. As clinical evidence demonstrated safety and efficacy, usage expanded. Improvements in polymer science enhanced implant strength and controlled absorption rates, supporting wider clinical acceptance.
Sources
Primary Research Interviews:
ENT Surgeons
Otolaryngologists
Medical Device Engineers
Hospital Procurement Heads
Clinical Researchers
Databases:
FDA Medical Device Database
PubMed
MedTech Europe
Magazines:
ENT Today
Medical Device Network
MedTech Dive
Diagnostics World,
Healthcare Design Magazine
Journals:
Laryngoscope Journal
Otolaryngology–Head and Neck Surgery
American Journal of Rhinology
Clinical Otolaryngology
ENT Journal
Newspapers:
Reuters Health
Wall Street Journal (Health)
Financial Times (MedTech)
The Guardian (Health)
The Hindu (Science)
Associations:
American Academy of Otolaryngology
European Rhinologic Society
International Federation of Oto-Rhino-Laryngological Societies
ENT UK
American Medical Association
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients