Uterine Fibroids Treatment Drugs Market Size and Trends
The uterine fibroids treatment drugs market is estimated to be valued at USD 2.02 Bn in 2025 and is expected to reach USD 3.49 Bn by 2032, growing at a compound annual growth rate (CAGR) of 8.1% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
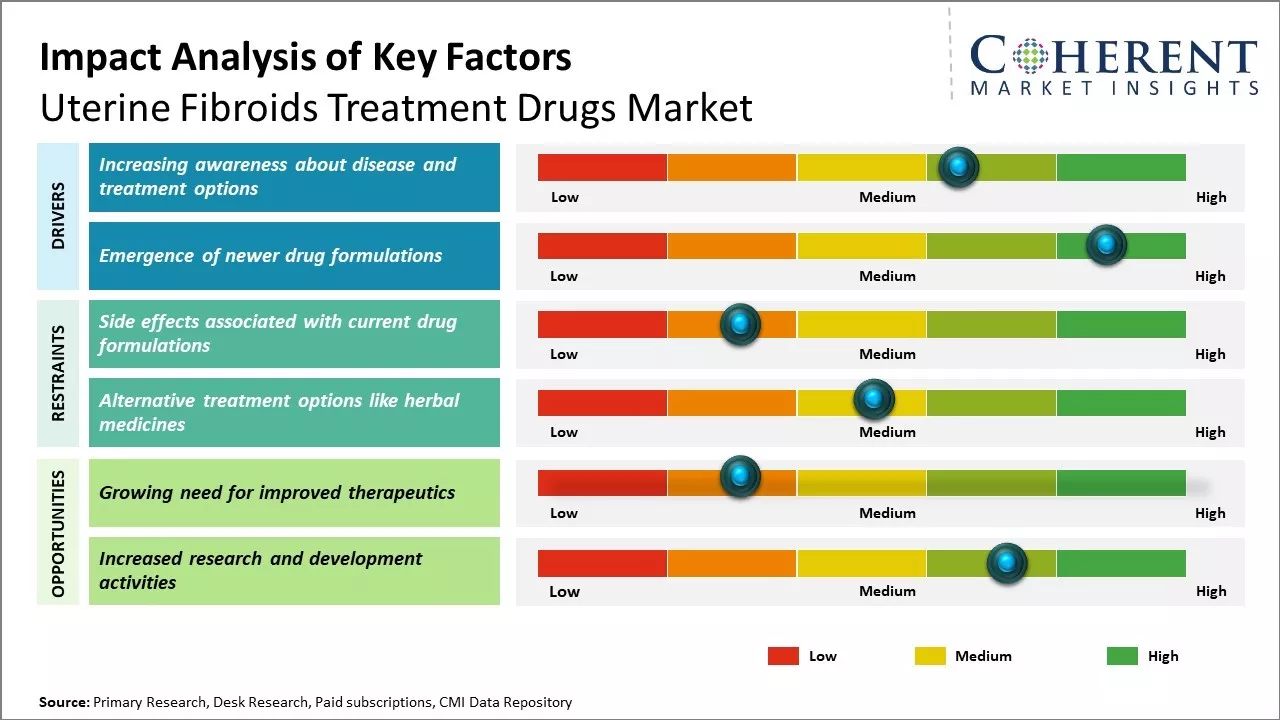
Over the past decade, the uterine fibroids treatment drugs market has witnessed steady growth. Key factors, such as the rising prevalence of uterine fibroids among women, growing awareness about disease treatment, and availability of various treatment options, have been driving the market growth. However, high treatment costs and side effects associated with long-term usage of drugs are expected to hamper the market growth. Meanwhile, the emergence of innovative drugs with better efficacy and safety is expected to create lucrative opportunities for market players over the coming years.
Increasing awareness about disease and treatment options
With greater awareness about uterine fibroids, its signs and symptoms, and available treatment options, more women are seeking medical help for managing this common condition. Various government and non-government organizations regularly conduct awareness programs and campaigns to educate women on fibroids and the various management approaches. They provide information about the different types and severity levels of fibroids, ways to diagnose fibroid types, and suitable treatment paths. This enables women to understand their condition better and make informed decisions. Also, with widespread access to internet and health portals, patients can easily lookup disease conditions and therapies. Drug makers too run branded awareness initiatives to promote their approved drugs. As more women get informed about this widespread women’s health issue that can impact quality of life, the demand for prescription medications and minimally invasive procedures is growing. This awareness tailwind is working in favor of the uterine fibroids treatment drugs market.
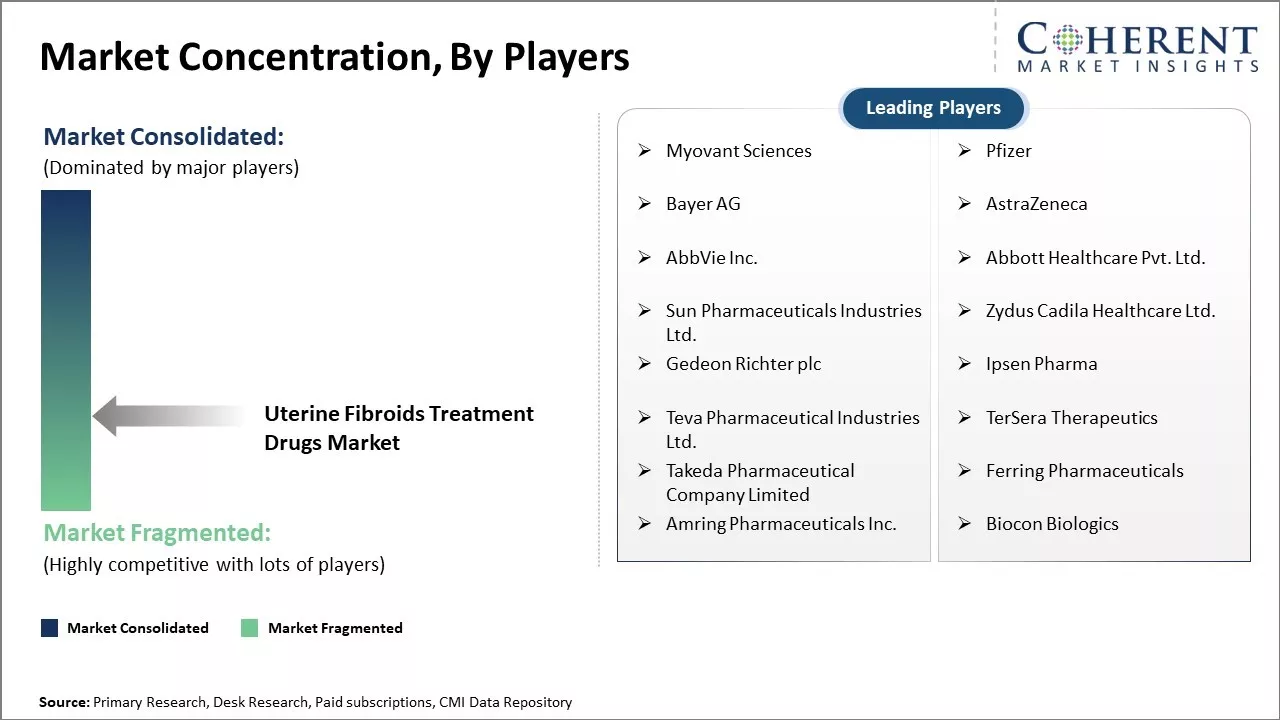
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Emergence of newer drug formulationsUterine fibroids remain a highly undertreated condition, mainly because currently available drug options have limitations like side effects and poor response rates. This has fuelled intensive R&D by pharmaceutical firms to develop safer and more targeted medications. In the last few years, a promising pipeline of novel drug formulations has emerged that can potentially address the deficiencies in existing therapies. These include selective progesterone receptor modulators, Gonadotropin hormone-releasing hormone (GnRH) antagonists with shorter flare protection, and other innovative targeting agents. Some of these new drugs have demonstrated encouraging results in terms of symptom relief, shrinkage of fibroid size, and quality of life improvements. With their approval and commercial availability, doctors and patients now have access to improved treatment alternatives. This is spurring higher prescription volumes and greater market demand for uterine fibroids drugs overall. The launch of better tolerated new drugs with superior efficacy can sustain the high growth momentum of this therapeutic category over the coming years.

To learn more about this report, Download Free Sample
Market Challenges: Side effects associated with current drug formulationsThe uterine fibroids treatment drugs market faces several challenges. Existing drug therapies are often inadequate and can cause unpleasant side effects, resulting in low patient compliance. The exact causes and risk factors for uterine fibroids are still not fully understood, making drug development difficult. Generic competition has eroded sales and margins of older branded drugs. The treatment landscape is also complex, as fibroids often require alternative approaches like minimally invasive procedures or surgeries in addition to pharmacological management.
Market Opportunities: Growing need for improved therapeutics
As the severity of fibroids and demand for non-invasive options increase, the need for improved therapeutics remains high. A growing understanding of fibroid pathogenesis could enable more targeted therapies. Partnerships between pharmaceutical companies and MedTech/diagnostics firms allow for comprehensive solutions. Rising healthcare access and spending in emerging markets also create new avenues for manufacturers to expand their business operation in developing market thereby, creating lucrative opportunities for market development over the forecasted period.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
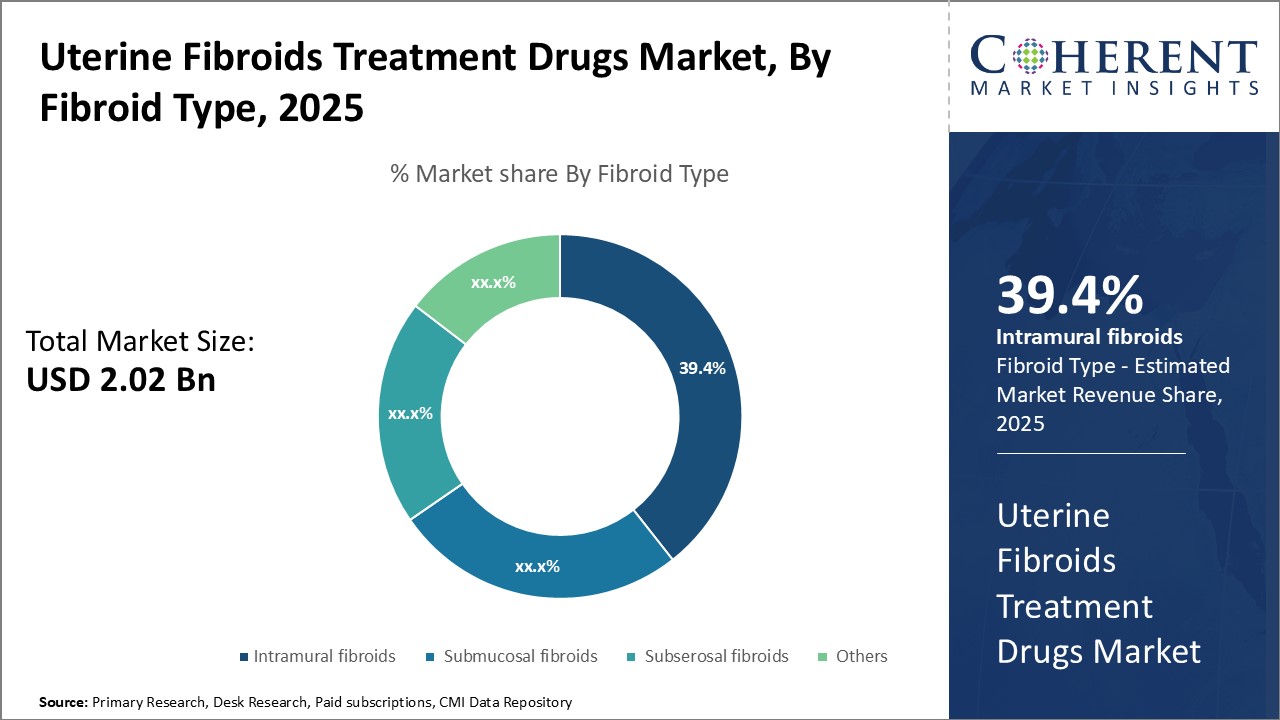
Insights, By Fibroid Type: Growing prevalence of intramural fibroidsThe fibroid type segment includes intramural fibroids, submucosal fibroids, subserosal fibroids, and others. Intramural fibroids segment is anticipated to hold 39.4% of the market share in 2025. Intramural fibroids develop within the muscular walls of the uterus. Given their internal location, these fibroids cause significant pain and discomfort through direct pressure and distortion of the uterine cavity. Women with large intramural fibroids often experience chronic pelvic pain, painful periods, backache, pain during sexual intercourse, frequent urination, difficulty emptying the bladder, bloating, and abdominal swelling. The painful symptoms substantially reduce quality of life and ability to perform daily activities. This drives many women to actively seek effective treatment options. The GnRH agonists and antagonists are primarily prescribed for short term treatment to reduce fibroid volume and related pain before surgical procedures. Oral medications like progesterone and contraceptives are also favored due to convenience of administration and clinical efficacy in relieving pain. Non-hormonal analgesics are not adequate to counter the chronic severe pain. This makes intramural fibroids the leading growth driver for GnRH therapies and analgesics in the uterine fibroids drugs market.
Insights, By Drug Type: Progesterone and combined oral contraceptives considered as first line treatment options
The drug type segment includes gonadotropin-releasing hormone (GnRH) antagonists, gonadotropin-releasing hormone (GnRH) agonists, progesterone & contraceptives, hormonal medications, and others. Progesterone & contraceptives are anticipated to account for the highest share of the uterine fibroids treatment drugs market and is projected to hold 37.3% of the market share in 2025. Progesterone and combined oral contraceptives are first line treatment options due to their ease of oral administration through pills. This eliminates injections or invasive procedures required by other hormonal therapies like GnRH agonists. Strong compliance is ensured as tablets can be self-administered at home on a regular schedule. Progesterone and contraceptives are also very well-established with a good safety profile. They provide multi-functional benefits of relieving fibroid symptoms and simultaneous contraception. This allows addressing two clinical needs with a single treatment choice. Additionally, progesterone-only pills counter abnormal bleeding whereas combined pills regulate menstrual cycles. These unique therapeutic features and convenience gains have led to wide physician recommendation and patient preference for progesterone and contraceptive drugs over injectable therapies. Their enduring market-leading position is underpinned by solid compliance on the back of simple oral dosing coupled with strong efficacy and comprehensive symptom-relieving action.
Insights, By Distribution Channel: Growing
The distribution channel segment includes hospital pharmacies, retail pharmacies, and online pharmacies. Retail pharmacies contributes the highest share of the uterine fibroids treatment drugs market and is projected to hold 42.1% of the market share in 2025. Women can walk into any neighborhood retail pharmacy and obtain uterine fibroids prescriptions conveniently without advance scheduling. This independence to fill scripts on demand from local pharmacies situated close to homes/offices fulfills the need for prompt therapy. Any delays in treatment are least preferred given debilitating impacts of fibroid symptoms on daily lives. Additionally, doctors often suggest specific pharmacies when writing prescriptions which, in turn, impacts where patients ultimately receive their medications from. Since the majority of physicians are more familiar with and hold accounts with select retail chains, they steer prescriptions in favor of those partnerships. Finally, many health plans also offer higher reimbursement at network retail pharmacies versus online options. These collective factors of same-day fulfillment, physician referral dominance, and insurance coverage superiority give retail pharmacies an upper hand over alternate channels in the competitive uterine fibroids treatment drug distribution market.
Regional Insights
Need a Different Region or Segment? Download Free Sample
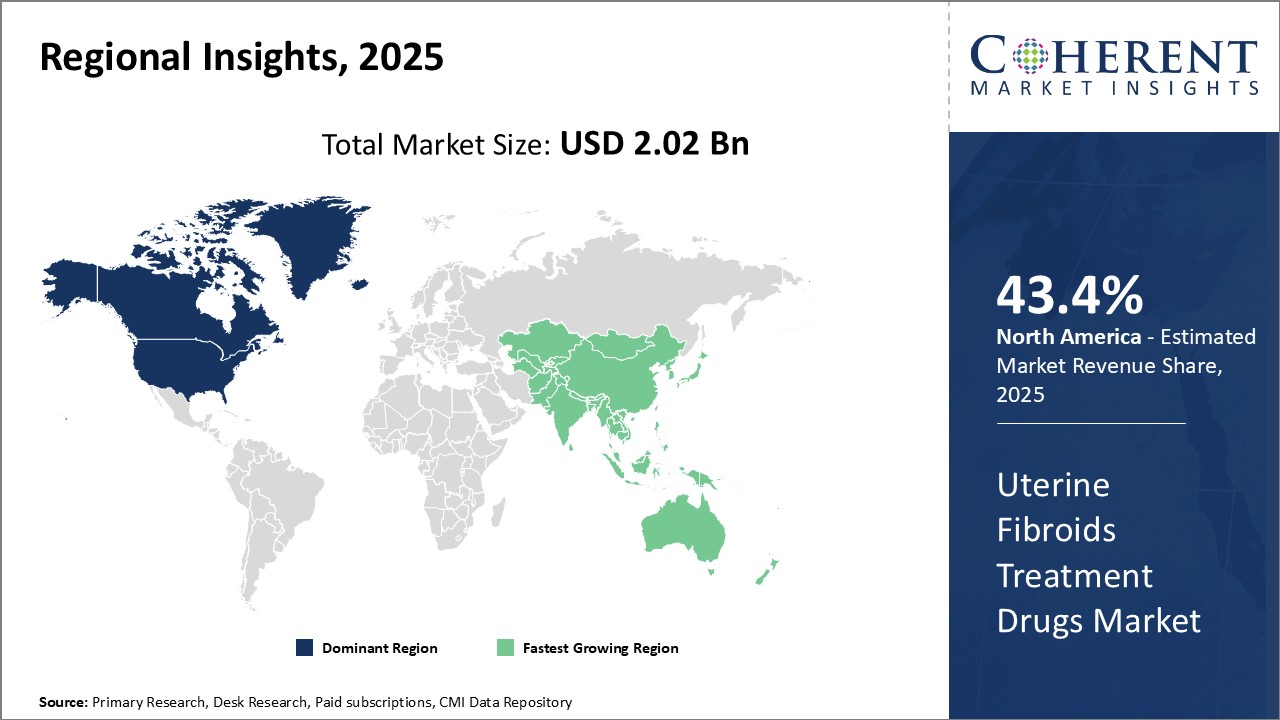
North America has dominated the global uterine fibroids treatment drugs market and is expected to hold 43.4% of the market share in 2025. This can be attributed to the advanced healthcare infrastructure and increasing awareness about various treatment options for uterine fibroids in the region. Major players have their headquarters located in countries like the U.S. and Canada which is driving extensive research and development activities for new and better treatment drugs. Further, favorable regulations encourage clinical trials, new product launches, and market presence of international manufacturers in the region.
However, Asia Pacific is identified as the fastest growing regional market led by countries like China and India. This growth can be contributed to the improving access to healthcare facilities in developing nations and rising medical tourism. Asia Pacific also comprises a huge patient population pool suffering from uterine fibroids due to increasing environmental pollution and changing lifestyle factors. International drug makers are focusing on the region by establishing production and distribution networks to tap into this burgeoning demand. Additionally, growing per capita incomes have boosted the spending capabilities for advanced treatment options.
Market Report Scope
Uterine Fibroids Treatment Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.02 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: | USD 3.49 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Myovant Sciences, Pfizer, Bayer AG, AstraZeneca, AbbVie Inc., Abbott Healthcare Pvt. Ltd., Sun Pharmaceuticals Industries Ltd., Zydus Cadila Healthcare Ltd., Gedeon Richter plc, Ipsen Pharma, Teva Pharmaceutical Industries Ltd., TerSera Therapeutics, Takeda Pharmaceutical Company Limited, Ferring Pharmaceuticals, Amring Pharmaceuticals Inc., and Biocon Biologics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Uterine Fibroids Treatment Drugs Industry News
- In October 2023, Sumitomo Pharma Canada, Inc., an innovative and entrepreneurial health care company, and Pfizer Canada, a pharmaceutical company announced that Health Canada has issued a Notice of Compliance (NOC) for MYFEMBREE (Relugolix, estradiol, and norethindrone acetate tablets) for the treatment of moderate to severe endometriosis pain in premenopausal women on October 17, 2023. On September 22, 2023, Health Canada authorized MYFEMBREE as a therapy for heavy menstrual bleeding caused by uterine fibroids in premenopausal women. This combination therapy oral drug, which is used once daily, provides women with a new option for treating endometriosis and uterine fibroids symptoms.
- In June 2022, ObsEva SA, a biopharmaceutical company, announced that the UK Medicines and Healthcare Products Regulatory Agency (MHRA) has approved Yselty (linzagolix), an oral GnRH antagonist, for the treatment of moderate to severe uterine fibroids (UF) in adult women of reproductive age (over 18 years old).
- In May 2021, Myovant Sciences, a biopharmaceutical company, and Pfizer Inc., a pharmaceutical and biotechnology corporation, announced that the U.S. Food and Drug Administration (FDA) approved MYFEMBREE (relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg), the first once-daily treatment for heavy menstrual bleeding associated with uterine fibroids in premenopausal women, with a treatment duration of up to 24 months.
- In May 2020, AbbVie, a pharmaceutical firm, and Neurocrine Biosciences, Inc, a biopharmaceutical business, announced that the U.S. FDA approved ORIAHNN (elagolix, estradiol, and norethindrone acetate capsules; elagolix capsules) for a treatment period of up to 24 months. ORIAHNN is the first non-surgical, oral medicine licensed by the U.S. FDA for managing heavy monthly bleeding caused by uterine fibroids in premenopausal women.
*Definition: The uterine fibroids treatment drugs market consists of pharmaceutical treatments that help reduce fibroid growth and symptoms. This market includes various drug types like hormonal therapy, anti-inflammatory drugs, contraceptives, and other medications. The drugs aim to shrink fibroids, prevent new growth, relieve pain and heavy bleeding caused by fibroids, and potentially delay or avoid surgery. The goal of the drugs in this market is to non-surgically manage and treat uterine fibroids.
Market Segmentation
- Fibroid Type Insights (Revenue, USD BN, 2020 - 2032)
- Intramural fibroids
- Submucosal fibroids
- Subserosal fibroids
- Others
- Drug Type Insights (Revenue, USD BN, 2020 - 2032)
- Gonadotropin-releasing hormone (GnRH) antagonists
- Gonadotropin-releasing hormone (GnRH) agonists
- Progesterone & Contraceptives
- Hormonal Medications
- Others
- Distribution Channel Insights (Revenue, USD BN, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD BN, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Myovant Sciences
- Pfizer
- Bayer AG
- AstraZeneca
- AbbVie Inc.
- Abbott Healthcare Pvt. Ltd.
- Sun Pharmaceuticals Industries Ltd.
- Zydus Cadila Healthcare Ltd.
- Gedeon Richter plc
- Ipsen Pharma
- Teva Pharmaceutical Industries Ltd.
- TerSera Therapeutics
- Takeda Pharmaceutical Company Limited
- Ferring Pharmaceuticals
- Amring Pharmaceuticals Inc.
- Biocon Biologics
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
